Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Yongfu, LIU Jianguo, ZHANG Litao
- PSI-driven cyclic electron flow partially alleviates the peroxidation of red alga Gelidium amansii (Gelidiaceae) caused by temporary high temperature
- Journal of Oceanology and Limnology, 40(1): 206-215
- http://dx.doi.org/10.1007/s00343-021-0452-z
Article History
- Received Nov. 23, 2020
- accepted in principle Jan. 7, 2021
- accepted for publication Mar. 11, 2021
2 Marine Biology and Biotechnology Laboratory, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
Gelidium amansii, a highly used commercial red seaweed that produces the finest quality agar, is widely distributed along the east coast of China (Huang, 2010). To date, the large-scale cultivation of this alga has not been successful, despite the many efforts to cultivate it in a variety of places (Li et al., 2019). The supply of Gelidium resources can only be collected from wild habitats, resulting in a significant decline of the wild stocks.
The zonation pattern of seaweed has been proven to be related to their physiological responses to environmental factors, including nutrient level, temperature, light intensity, and desiccation (Zaneveld, 1969; Davison and Pearson, 1996). Typically, G. amansii is considered to be a subtidal, perennial red seaweed that primarily grows on calcareous substrate in subtidal waters (Lee and Shiu, 2009; Xie and Sun, 2018). Our surveys along the Jiaozhou Bay coast during 2016–2018 showed that G. amansii could also survive in the tidal pools that were 0.3-m deep where the water temperature can reach as high as 30 ℃ during low tides in summer (Li et al., 2019). During the survey, samples of macroalgae were collected for identification in the supratidal zone, intertidal zone, and subtidal zone with water depth < 1 m at the sampling section at the lowest tide of each season. The tidal pools are about 2–5 m in size, and are not affected by tide (i.e. the whole pond is exposed to the air) during the 2 h of the low tide. We sought to understand the mechanism that ensures survival of G. amansii in these sites. The performance traits and tolerance of algae under different temperatures are two principal aspects determining the natural distribution and cultivation prospects of seaweed, especially the economic ones (Eggert, 2012). Understanding the responses and possible mechanisms for acclimation to low or high temperature is crucial for a sustainable seaweed cultivation industry, e.g., Kappaphycus (Li et al., 2016a, b; Kumar et al., 2020) and Gracilaria (Yang et al., 2015). The influence of temperature on the algae is time dependent, i.e. the rules of short-term physiological regulation and long-term acclimation or adaptation of algae is often different (Eggert, 2012). It involves whether the damage caused by temperature is recoverable (Sampath-Wiley et al., 2008; Blouin et al., 2011). For high temperature, thermal stress may not be enough to cause harm to organisms in a very short time scale, but long-term effects irreversibly reduce growth. Generally, temperature optima of photosynthesis are situated well above the temperature optima of growth (Eggert and Wiencke, 2000). This shows that temperature effects on a specific physiological process (i.e., photosynthesis in this case) do not necessarily correspond to the temperature-growth pattern as growth integrates the effect of temperature on the total metabolism. However, there is also useful information to be gained by investigating the photosynthetic response to temperature. Photosynthesis is the most temperature-sensitive process in plants and growth temperature is a key factor as it determines the CO2 fixation capacity as well as the activity of photosynthetic apparatus (Sharkey and Schrader, 2006; Mathur et al., 2014; Brestic et al., 2018). More conveniently, the photoreaction activity can be detected non-invasively by chlorophyll fluorescence probe, as many researches adopted nowadays (Alemu, 2020).
In our previous study, we also reported that the optimal temperature for photosynthetic oxygen evolution of G. amansii ranged from 22 ℃ to 26 ℃, which is consistent with the previous results obtained based on growth performance (Xie and Sun, 2018). Exposure to 30 ℃ induced significant losses of chlorophyll a and carotenoids and an increased phycobiliproteins content. The carbon assimilation process, light harvesting pigments, PSII reaction centers, and PSII acceptor side of G. amansii were damaged under 30 ℃ at 12 h; however, the PSII donor side (oxygen-evolving complex, OEC) was relatively stable during the initial 12-h treatment. Previous studies on other algae and higher plants, e.g., Arthrospira sp. (Zhang and Liu, 2016), wheat Triticum aestivum (Mathur et al., 2011a, b), and potato Solanum tuberosum (Dou et al., 2014) have suggested that high-temperature stress may lead to the dissociation of OEC, resulting in an imbalance between the electron flow from OEC to the PSII acceptor side in the direction of the PSI reaction center (De Ronde et al., 2004). This interesting phenomenon further encouraged us to investigate the protective mechanism of G. amansii against photoinhibition under high temperature stress.
In some seaweeds, CEF-I has been found to be a key photo-protective mechanism under various environmental stresses (Larkum et al., 2017). The assimilation of one CO2 molecule consumes three ATP and two NADPH molecules in one cycle of the Calvin-Benson cycle. Meanwhile, only 2.6 ATP are generated when 2 molecules of NADPH are produced in linear electron flow. This means a shortfall of 0.4 ATP for sustained operation of the Calvin-Benson cycle (Yamori and Shikanai, 2016). As an important alternative electron flow, PSI-driven cyclic electron flow (CEF-I) is essential for photosynthesis in many higher plants and algae. In CEF-I, electrons are recycled from the stromal reducing pool to the plastoquinone (PQ) pool, generating ΔpH and consequently ATP without accumulation of NADPH (Shikanai, 2007). The CEF-dependent generation of proton gradient across thylakoid membranes is considered to be the main way to increase the ATP/NADPH ratio as well as to protect the photosystem from photoinhibition through activating non-photochemical quenching and stabilizing OEC (Shikanai, 2007, 2016; Takahashi et al., 2009; Huang et al., 2012; Wang et al., 2015). CEF-I includes NAD(P)H dehydrogenase complex (NDH) and PGR5 (Proton Gradient Regulation 5) /PGRL1 (Proton Gradient Regulation Like 1) pathways. Among them, PGR5/PGRL1-dependent CEF is considered to be the key pathway in C3 plants (Huang et al., 2018), which can be specifically inhibited by Antimycin A (AA) (Shikanai, 2007). Under high temperature stress, the enhanced photorespiration resulted in an increased of ATP demand. CEF-I is activated to increase ATP supply. Meanwhile, HCO3- influx across cell membranes in the CO2 concentrating mechanism (CCM) of algae is also ATP-required (Price, 2011). CCM further intensifies the CO2 demand for cell photosynthesis. Aihara et al. (2016) have reported that temperatures above the normal tolerance range induced CEF-I in Symbiodinium. Moreover, the induction of reactive oxygen species (ROS) and oxidative stress by high temperature has also been observed in macroalgae (Lee and Shiu, 2009; Eggert, 2012). The antioxidant system is the main way for plants to remove ROS. Antioxidant enzymes induced by heat stress could help to ameliorate the dramatic increase in oxidative stress (Guo et al., 2006). Information derived from the measurement of ROS and antioxidant enzymes may aid in understanding influence of temporary high-water temperatures on this seaweed.
In this study, the variations of CEF-I and antioxidant enzymes of G. amansii before and after treatment at high temperature were recorded to investigate the possible protective effect of CEF-I on photosynthesis of this alga.
2 MATERIAL AND METHOD 2.1 Plant material and pretreatmentGelidium amansii that inhabits the Second Beach, Qingdao, China was collected for the experiment in which algae were exposed to high temperature. Samples were collected at a depth of about 1 m at low tide. Sixty healthy individuals (without showing signs of disease and discoloration on the thalli) were used for the experiment. Collected algae were kept in seawater using an ice cooler at about 15 ℃ and transported to the laboratory within a half hour. Before the treatment, algae that had been carefully rinsed with fresh filtered seawater (salinity 32) were placed in a growth chamber with 16-L modified f/2 medium (without pH adjustment, composition of the elements were shown in Li et al. (2016c) for 5 days under natural lighting. The water temperature in the tank was controlled at 20 ℃. Based on the identification method provided by Huang (2010), tetrasporophytes of this alga were used for the next heat treatment.
2.2 Analytical procedureGlass beakers containing 400-mL fresh seawater were placed into an incubator at 30 ℃ for 12 h with continuous low light (LL) 40 μmol photons/(m2·s) and continuous high light (HL) with 1 000 μmol photons/(m2·s), respectively. Samples treated at 20 ℃ for the same period were utilized as controls. Healthy fronds (each 2.0 g, 3–4 cm in height) were chosen as test samples. Four individuals were placed in the incubator as a parallel sample. Samples treated for 6 h and 12 h at 20 and 30 ℃, respectively, were used to determine the chlorophyll fluorescence and maximum light utilization efficiency of PSII (Fv/Fm) and other photosynthetic parameters, i.e., qP, Fv'/Fm', and ΦPSII, representing the photochemical quenching, light adapted maximum quantum yield of PSII, and PSII effective quantum yield, respectively, were used to reflect the activity of photoreaction as described by Zhang et al. (2011). The measurement for each treatment was repeated for four times with dark-adapted (for 15 min) leaf disks at room temperature. Briefly, the initial fluorescence F0 of fronds was recorded in low modulated measuring light. Next, a 0.7-s pulse of saturating light (8 000 μmol photons/(m2·s)) was applied to detect Fm (the maximum fluorescence). The difference between Fm and F0 is the variable fluorescence, abbreviated as Fv. The steady-state fluorescence level (Fs) and the maximum chlorophyll fluorescence level (Fm') during exposure to illumination with actinic light at 40 μmol photons/(m2·s) were also measured, respectively. Fm/F0, an indicator of linear electron transfer rate, was calculated as previously described (Ibrahim and Jaafar, 2012). Fv/F0, the ratio of variable fluorescence to unquenchable portion of fluorescence was also determined to indicate activity of photosynthetic reaction centers (González-Mendoza et al., 2013; Kula et al., 2017). In an attempt to display the response of photosynthesis to high (30 ℃) and standard temperatures (20 ℃), the parameters Fv/Fm, Fv/F0, Fm/F0, qP, Fv'/Fm', and ΦPSII in the thalli that had been treated for 6 and 12 h under LL condition were also recorded.
The post illumination increase in the intensity of chlorophyll fluorescence was measured as described by Wang et al. (2006) and Zhang et al. (2017). Before the measurements, the four 6 h heat-treated fronds of specific conditions (20 ℃-LL, 20 ℃-HL, 30 ℃-LL, and 30 ℃-HL, respectively) were combined as a single sample and adapted to dark for 15 min at same temperature as that of exposure. The transient post illumination was recorded after termination of the illumination by white actinic light with wavelength 400–750 nm for 2 min (AL, 40 μmol photons/(m2·s) and 1 000 μmol photons/(m2·s), respectively) (Fig. 1a). The post illumination increase was quantitatively described using the enhancement of chlorophyll florescence (ΔF) and initial rate (S) after the AL termination.


|
| Fig.1 The post illumination increase in chlorophyll florescence of Gelidium amansii exposed to high temperatures under low (LL, 40 μmol photons/(m2·s)) and high (HL, 1 000 μmol photons/(m2·s)) light for 6 h Four treated fronds of specific conditions (20 ℃-LL, 20 ℃-HL, 30 ℃-LL, and 30 ℃-HL) were combined as a single sample and then used to determine the florescence intensity. AL: white actinic light. In (a), a typical chlorophyll fluorescence curve can be observed when the AL is provided (from AL on to about 220 s). When AL was removed (AL off), an instantaneous enhancement of chlorophyll fluorescence intensity occurred. The fluorescence intensity after AL removal are recorded and analyzed to quantify the PSI-driven cyclic electron flow. |
where Fmax and Fmin are the maximum and minimum fluorescence intensity after the AL treatment, respectively; t represents the time between Fmax and Fmin. S was obtained by fitting the data using a linear least square method based on Origin 8.0 (OriginLab, Northampton, MA, USA).
Antimycin A (AA) is a specific inhibitor of CEF-I (Shikanai, 2007). To monitor the CEF-I, 10 μmol/L of AA was used to incubate with algal fronds pre-treated at 30 ℃ for 6 h.
Fronds treated at high temperature for 6 h and 12 h, respectively, were used to determine the antioxidant enzyme system. The activities of ascorbate peroxidase (APX), superoxide dismutase (SOD), and catalase (CAT), and content of malonaldehyde (MDA) were determined using an ascorbate peroxidase assay kit, a total superoxide dismutase assay kit, a hydroperoxide dehydratase assay kit, and a malondialdehyde assay kit, respectively, provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) as described by Li et al.(2016a, b).
Differences between the measurements were analyzed using a one-way ANOVA complemented by Ducan post hoc test using the program SPSS 17.0 (IBM, Inc., Armonk, NY, USA). Statistical significance was evaluated at a probability level of P < 0.05.
3 RESULT 3.1 Heat-induced changes on cyclic electron flow at different light intensitiesWhen AL was removed, an instantaneous enhancement of chlorophyll fluorescence intensity occurred (Fig. 1a). The quantitative result of this visible increase showed that when the algae were treated at 30 ℃, both the ΔF and S were enhanced, under either LL or HL conditions (Fig. 1b–e; Table 1). Otherwise, the CEF-I can be decreased by AA (Fig. 2). After treatment with10 μmol/L of AA for 5 min, the enhanced CEF-I at 30 ℃ with high light decreased to the level of that at 20 ℃ with the same light intensity (Table 1).

|

|
| Fig.2 The post illumination increase in chlorophyll florescence of Gelidium amansii exposed to high temperature under high light that can be further treated using 10 μmol/L of inhibitor antimycin A for 5 min AA: antimycin A; HL: high light. Four treated fronds under 30 ℃-HL were combined as a single sample and then used to determine the florescence intensity. |
It has been widely proven that chlorophyll fluorescence is a convenient and particularly noninvasive probe to evaluate the photosynthetic activity under various abiotic stresses (Baker, 2008). With the goal of exploring effect of high temperature on the photosynthesis of G. amansii, chlorophyll fluorescence parameters of algae treated at 30 ℃ for different periods are shown in Fig. 3. Fv/Fm, Fv/F0, and Fm/F0 all decreased with the time of exposure. However, the lowest decrease was all recorded at 6 h, with the degrees of 19%, 40%, and 25%, respectively, compared with those of the control. At 12 h, these three parameters decreased by more than 54%, 76%, and 48%, respectively, in comparison with the control. With regard to Fv'/Fm' and ΦPSII, the values were both relatively stable during the initial 6 h period and then decreased sharply at 12 h. qP reflects the share of light energy absorbed by antenna pigments used for photochemical reactions, i.e., the fraction of open PSII centers (Várkonyi et al., 2005).

|
| Fig.3 The variations of chlorophyll fluorescence parameters in fronds of Gelidium amansii at 30 ℃ with light intensity of 40 μmol photons/(m2·s) for different times (i.e., 6 h and 12 h) Samples treated at 20 ℃ with 40 μmol photons/(m2·s) for the same period of time were utilized as controls. Values are the averages of four biological replicates±SD. The data of each parameter with different letters are significantly different (P < 0.05). Each replicate is from an individual piece of thallus. |
Fv/Fm is an indicator of photoinhibition in plants (Takahashi et al., 2009; Zhang et al., 2015). It is indicated that HL induces a significant inhibition of G. amansii at 20 ℃ (Fig. 4a). Exposure at 30 ℃ also reduced the Fv/Fm of this alga, either under low light or high light conditions (Fig. 4b & c). These reductions were further intensified when the inhibitor of CEF-I was added. This phenomenon suggests that CEF-I may alleviate the photoinhibition caused by high temperature or strong light.
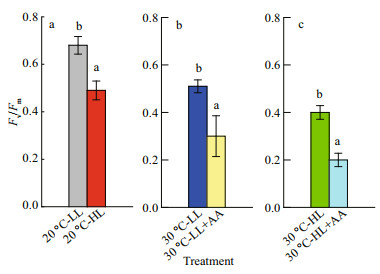
|
| Fig.4 The changes of maximal photochemical efficiency of PSII (Fv/Fm) of Gelidium amansii exposed to different temperatures with low and high light Values are the averages of four biological replicates±SD. The data of each parameter with different letters are significantly different (P < 0.05). AA: antimycin A; HL: high light; LL: low light. |
Heat treatment resulted in a similar response of the activities SOD, APX, and CAT, and the contents of H2O2 and MDA. Each parameter increased slightly during the first 6 h of exposure at 30 ℃, with the exception that MDA remained stable as shown in Fig. 5. At 12 h, all the indices increased significantly.

|
| Fig.5 The variation in H2O2 concentrations, activities of superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX), and contents of malondialdehyde (MDA) in Gelidium amansii exposed to 30 ℃ with 40 μmol photons/(m2·s) for different times Samples treated at 20 ℃ with 40 μmol photons/(m2·s) for the same period were utilized as control. No significant changes of these parameters in the algae treated at 20 ℃ for 0, 6, and 12 h were observed. Thus, only the data of the algae that were treated at 30 ℃ are shown here. Values are the averages of three replicates±SD. The data of each parameter with different letters are significantly different (P < 0.05). |
CEF-I, as an electron flow pathway in the thylakoid membranes of algae, has proven to help intertidal algae resist abiotic stress. For example, Ulva (Gao et al., 2011) and Porphyra (now Pyropia) yezoensis (Gao and Wang, 2012) are resistant to desiccation stress, Sargassum fusiforme to saline stress (Huan et al., 2014; Gao et al., 2016), and Pyropia yezoensis to high light (Niu et al., 2016). In higher plants, studies conducted using tobacco (Huang and Hu, 2015) and spinach (Agrawal et al., 2016) leaves have confirmed that the CEF-I can protect PSI and PSII from high temperature damage. There is little information regarding the CEF-I of subtidal macroalgae, particularly with respect to the response of this physiological process to high temperature. As shown in Fig. 1a, a transient increase in post illumination when the AL was removed can also be observed in G. amansii. Such occurrence can be attributed to the reduction of plastoquinone (PQ) mediated by reducing substances that have accumulated in the presence of incident light (Muraoka et al., 2006; Wang et al., 2006). ΔF and S are used to quantitatively describe the intensity of CEF-I. After AA treatment, these two parameters were both significantly lowered, suggesting that AA is a potent inhibitor of CEF-I in G. amansii and can be used to measure the involvement of CEF-I in photosynthetic reaction. The PGR5/PGRL1-dependent CEF-I can be specifically inhibited by AA (Shikanai, 2007). We further believe that PGR5/PGRL1-dependent CEF-I at least is present in G. amansii. To the best of our knowledge, this is the first study that confirms the existence of CEF-I in G. amansii (Fig. 1a).
In our previous study, we reported that the oxygen-evolving complex (OEC) was stable at 30 ℃ stress for 6 h (Li et al., 2019). The results of this study further indicate that the photochemical efficiency of PSII, the size and the number of active photosynthetic reaction centers (as indicated by Fv/F0), and the linear electron transfer rate (as indicated by Fm/F0) are all gradually inhibited by high temperature. Interestingly, both the maximal photochemical efficiency under light (Fv'/Fm') and maximum quantum yield of PSII after light adaptation (ΦPSII) changed only slightly during initial 6 h. ΦPSII is multiplied by qP and Fv'/Fm'. qP decreased slightly throughout the high temperature treatment for 12 h, suggesting that the lowered ΦPSII (from 0.54 to 0.13) was primarily a result of decrease in Fv'/Fm' at 12 h. However, all the parameters, i.e. ΦPSII, Fv'/Fm', and qP, changed little when the fronds treated at 30 ℃ for 6 h. Presumably, there is a physiological process that dissipated the light energy absorbed excessively during the first 6 h and helped G. amansii maintain a relatively high maximum photochemical efficiency under light. Our results showed that CEF-I plays an important role in mitigating the heat damage of G. amansii caused by high temperature. As shown in Fig. 1b–e and Table 1, both the initial rate of the increasing phase and the amplitude were up-regulated in the G. amansii that had been exposed to high temperature, either at low or high light intensity. This leads to the determination of possible cause of inconsistent response between theoretical maximum value after dark adaptation and that of the actual value measured under light. It is highly likely that the light-activated CEF-I relieves the pressure of photosynthetic system caused by high temperature and protects OEC from temporary heat damage (Huang et al., 2015; Yang et al., 2017).
A transient post illumination increase in the fluorescence intensity of chlorophyll is considered to arise from reduction of PQ (Burrows et al., 1998; Kofer et al., 1998). Two alternative pathways have been demonstrated for the PSI-driven CEF pathway. The main pathway is mediated by PGR5, a proton gradient regulation protein (Munekage et al., 2002). The second pathway is mediated by the NADH dehydrogenase (NDH) complex, a homolog of mitochondrial complex I (Johnson, 2011). AA is typically a potent inhibitor of CEF in those organisms that have a strong component of PGR- or NDH-dependent CEF, and where it is active, it can be used to measure the involvement of CEF in photosynthetic reactions (Endo et al., 1997). Both the ΔF and S of AA fed-thalli of G. amansii decreased to the levels of control (20 ℃-HL) at the end of 6 h of heat stress, suggesting that this inhibitor can be used to clarify the CEF in this alga. The addition of CEF-I inhibitors usually led to a significant increase in non-photochemical quenching, which implied that CEF-I was an essential ATP supply mechanism that used the energy absorbed by photosynthetic apparatus (Zhang et al., 2017). The qP of heat-exposed G. amansii maintained a stable level during the treatment period. However, the ΦPSII decreased at 12 h. It can be deduced that the reduction of photochemical efficiency is partly attributed to the up-regulated non-photochemical quenching, i.e., heat dissipation (data not shown) (Yin et al., 2010; Naeem et al., 2020). Our previous study showed that the OEC indicates a high tolerance to 30 ℃ stress within 12 h. This study provides an explanation for this phenomenon. The stabilization of OEC requires a necessary transmembrane proton gradient (ΔpH) (Wang et al., 2006; Takahashi et al., 2009). The formation of ΔpH caused by increased CEF-I could suppress the damage of OEC and then alleviate the reduction of PSII activity. During heat stress, the drop of Fv/Fm in AA-fed fronds intensified significantly in comparison with that without the inhibitor, suggesting that the photoinhibition caused by heat stress was intensified when the CEF-I was down-regulated.
An oversupply of light energy accelerates the production of ROS and leads to occurrence of photoinhibition. Plants have evolved antioxidant enzyme systems (e.g., SOD, APX, and CAT), which can be used to remove ROS and thus avoid the damage of excessive light energy (Mittler, 2002). No significant change in MDA, a marker of lipoperoxidation, was found at 6 h, although the H2O2 content and antioxidant enzyme activity had increased (Fig. 5). At 12 h, all the antioxidant enzymes of heat-treated plants measured in this study were fully activated, resulting in a high level of H2O2 and a rapid increase in lipoperoxidation. It can be further deduced that the protective effect of CEF-I is not sufficient to resist the heat-induced damage of linear electron transfer in photosynthetic apparatus of G. amansii. Therefore, the high temperature of 30 ℃ is ultimately lethal to this alga. It should be noted that the results of this study are based on short-term laboratory measurements (on hours timescales) of CEF-I and antioxidant enzyme responses of G. amansii. We expect that the results are helpful in reflecting the acclimation of G. amansii to high temperature. Extrapolation of our results must be done with caution, and we acknowledge that studies on a longer timescale are needed to verify our inferences, especially in term of growth adaptation in different temperatures. Moreover, heat shock proteins (HSP) are present in organisms and its activation is triggered by high temperatures to prevent protein denaturation (Wang et al., 2004). Enhanced production and accumulation of free and conjugated polyamines as well as increased activities of their biosynthetic enzymes in plants also have been associated with heat stress (Kuznetsov et al., 2006). Nowadays, study on temperature effects on HSPs and polyamines of G. amansii still remains scarce and it is an interesting subject needs further study.
A semi-diurnal tide is the tidal type along the east coast of China (Teng et al., 2017), which is the area in which G. amansii is primarily distributed. This type of tide results in two high tides and two low tides every day. The difference between the first high tide and the next low tide is approximately 6 h. G. amansii that inhabits the intertidal rocky zones will not be exposed to high temperatures for less than 6 h, even at very low tides. These results showed that activated CEF-I partially protected the photosynthetic apparatus from high temperature that causes ROS damage after 6 h of exposure to high temperature. The up-regulated CEF-I is an important physiological process that enables G. amansii to adjust to fluctuating natural high temperature. It is still unclear whether there are other factors that dominate the geographical distribution of this species, such as the content of osmotic solutes and soluble proteins, e.g., proline, glycine betaine, and phycobiliprotein. This is an interesting subject that merits further study.
5 CONCLUSIONCEF-I was up-regulated in G. amansii exposed to 30 ℃, regardless of whether it was coupled with high or low light, compared with that grown at 20 ℃. The enhanced CEF-I alleviated the photoinhibition of PSII in this alga. The enhancement of CEF-I activity at high temperature aids in the generation of ΔpH and protects the donor side of PSII, i.e., oxygen-evolving complex, against heat damage. However, the protection of CEF-I is not sufficient for G. amansii to adapt to high temperature stress. Therefore, CEF-I is regarded as an important strategy by G. amansii to acclimate to high temperatures in a relatively short time.
6 DATA AVAILABILITY STATEMENTFull information developed from this study is available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTThe authors would like to thank Dr. Hu LI, Institute of Oceanology, Chinese Academy of Sciences, for his kind help in collecting the seaweed.
Agrawal D, Allakhverdiev S I, Jajoo A. 2016. Cyclic electron flow plays an important role in protection of spinach leaves under high temperature stress. Russian Journal of Plant Physiology, 63(2): 210-215.
DOI:10.1134/S1021443716020023 |
Aihara Y, Takahashi S, Minagawa J. 2016. Heat induction of cyclic electron flow around photosystem I in the symbiotic dinoflagellate Symbiodinium. Plant Physiology, 171(1): 522-529.
DOI:10.1104/pp.15.01886 |
Alemu S T. 2020. Photosynthesis limiting stresses under climate change scenarios and role of chlorophyll fluorescence: a review article. Cogent Food & Agriculture, 6(1): 1785136.
|
Baker N R. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology, 59: 89-113.
DOI:10.1146/annurev.arplant.59.032607.092759 |
Blouin N A, Brodie J A, Grossman A C, Xu P, Brawley S H. 2011. Porphyra: a marine crop shaped by stress. Trends in Plant Science, 16(1): 29-37.
DOI:10.1016/j.tplants.2010.10.004 |
Brestic M, Zivcak M, Hauptvogel P, Misheva S, Kocheva K, Yang X H, Li X N, Allakhverdiev S I. 2018. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynthesis Research, 136(2): 245-255.
DOI:10.1007/s11120-018-0486-z |
Burrows P A, Sazanov L A, Svab Z, Maliga P, Nixon P J. 1998. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. The EMBO Journal, 17(4): 868-876.
DOI:10.1093/emboj/17.4.868 |
Davison I R, Pearson G A. 1996. Stress tolerance in intertidal seaweeds. Journal of Phycology, 32(2): 197-211.
DOI:10.1111/j.0022-3646.1996.00197.x |
De Ronde J A D, Cress W A, Kruger G H J, Strasser R J, Staden J V. 2004. Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. Journal of Plant Physiology, 161(11): 1211-1224.
DOI:10.1016/j.jplph.2004.01.014 |
Dou H O, Xv K P, Meng Q W, Li G, Yang X H. 2014. Potato plants ectopically expressing Arabidopsis thaliana CBF3 exhibit enhanced tolerance to high-temperature stress. Plant, Cell & Environment, 38(1): 61-72.
|
Eggert A, Wiencke C. 2000. Adaptation and acclimation of growth and photosynthesis of five Antarctic red algae to low temperatures. Polar Biology, 23: 609-618.
DOI:10.1007/s003000000130 |
Eggert A. 2012. Seaweed responses to temperature. In: Wiencke C, Bischof K eds. Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization. Springer, Berlin, Heidelberg. p. 47-66.
|
Endo T, Mil H, Shikanai T, Asada K. 1997. Donation of electrons to Plastoquinone by NAD(P)H dehydrogenase and by Ferredoxin-Quinone reductase in Spinach chloroplasts. Plant and Cell Physiology, 38(11): 1272-1277.
DOI:10.1093/oxfordjournals.pcp.a029115 |
Gao S, Huan L, Lu X P, Jin W H, Wang X L, Wu M J, Wang G C. 2016. Photosynthetic responses of the low intertidal macroalga Sargassum fusiforme (Sargassaceae) to saline stress. Photosynthetica, 54(3): 430-437.
DOI:10.1007/s11099-015-0181-7 |
Gao S, Shen S D, Wang G C, Niu J F, Lin A P, Pan G H. 2011. PSI-driven cyclic electron flow allows intertidal macroalgae Ulva sp. (Chlorophyta) to survive in desiccated conditions. Plant and Cell Physiology, 52(5): 885-893.
|
Gao S, Wang G C. 2012. The enhancement of cyclic electron flow around photosystem I improves the recovery of severely desiccated Porphyra yezoensis (Bangiales, Rhodophyta). Journal of Experimental Botany, 63(12): 4349-4358.
DOI:10.1093/jxb/ers082 |
González-Mendoza D, Gil F F Y, Escoboza-Garcia F, Santamaría J M, Zapata-Perez O. 2013. Copper stress on photosynthesis of black mangle (Avicennia germinans). Anais Da Academia Brasileira de Ciências, 85(2): 665-670.
DOI:10.1590/S0001-37652013000200013 |
Guo Y P, Zhou H F, Zhang L C. 2006. Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Scientia Horticulturae, 108(3): 260-267.
DOI:10.1016/j.scienta.2006.01.029 |
Huan L, Xie X J, Zheng Z B, Sun F F, Wu S C, Li M Y, Gao S, Gu W H, Wang G C. 2014. Positive correlation between PSI response and oxidative pentose phosphate pathway activity during salt stress in an intertidal macroalga. Plant and Cell Physiology, 55(8): 1395-1403.
DOI:10.1093/pcp/pcu063 |
Huang L J. 2010. Artificial Culture of Gelidium amansii (Lamx.). 2nd edn. China Ocean Press, Beijing. 7-123.
|
Huang W, Hu H. 2015. Effect of growth temperature on the activity of cyclic electron flow in tobacco leaves. Plant Diversity and Resources, 37(3): 283-292.
(in Chinese with English abstract) |
Huang W, Yang Y J, Hu H, Zhang S B. 2015. Different roles of cyclic electron flow around photosystem I under sub-saturating and saturating light intensities in tobacco leaves. Frontiers in Plant Science, 6: 923.
|
Huang W, Yang Y J, Zhang S B, Liu T. 2018. Cyclic electron flow around photosystem i promotes ATP synthesis possibly helping the rapid repair of photodamaged photosystem ii at low light. Frontiers in Plant Science, 9: 239.
DOI:10.3389/fpls.2018.00239 |
Huang W, Zhang S B, Cao K F. 2012. Physiological role of cyclic electron flow in higher plants. Plant Science Journal, 30(1): 100-106.
(in Chinese with English abstract) DOI:10.3724/SP.J.1142.2012.10100 |
Ibrahim M H, Jaafar H Z E. 2012. Reduced photoinhibition under low irradiance enhanced Kacip Fatimah (Labisia pumila Benth) secondary metabolites, Phenyl alanine lyase and antioxidant activity. International Journal of Molecular Sciences, 13(5): 5290-5306.
DOI:10.3390/ijms13055290 |
Johnson G N. 2011. Reprint of: physiology of PSI cyclic electron transport in higher plants. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1807(8): 906-911.
DOI:10.1016/j.bbabio.2011.05.008 |
Kofer W, Koop H U, Wanner G, Steinmüller K. 1998. Mutagenesis of the genes encoding subunits A, C, H, I, J and K of the plastid NAD(P)H-plastoquinone-oxidoreductase in tobacco by polyethylene glycolmediated plastome transformation. Molecular and General Genetics MGG, 258(1-2): 166-173.
DOI:10.1007/s004380050719 |
Kula M, Kalaji H M, Skoczowski A. 2017. Culture density influence on the photosynthetic efficiency of microalgae growing under different spectral compositions of light. Journal of Photochemistry and Photobiology B: Biology, 167: 290-298.
DOI:10.1016/j.jphotobiol.2017.01.013 |
Kumar Y N, Poong S W, Gachon C, Brodie J, Sade A, Lim P E. 2020. Impact of elevated temperature on the physiological and biochemical responses of Kappaphycus alvarezii (Rhodophyta). PLoS One, 15(9): e0239097.
DOI:10.1371/journal.pone.0239097 |
Kuznetsov V V, Radyukina N L, Shevyakova N I. 2006. Polyamines and stress: biological role, metabolism, and regulation. Russian Journal of Plant Physiology, 53(5): 583.
DOI:10.1134/S1021443706050025 |
Larkum A W D, Szabó M, Fitzpatrick D, Raven J A. 2017. Cyclic electron flow in cyanobacteria and eukaryotic algae. In: Barber J, Ruban A V eds. Photosynthesis and Bioenergetics. World Scientific Publishing, Singapore. p. 305-343.
|
Lee T M, Shiu C T. 2009. Implications of mycosporine-like amino acid and antioxidant defenses in UV-B radiation tolerance for the algae species Ptercladiella capillacea and Gelidium amansii. Marine Environmental Research, 67(1): 8-16.
DOI:10.1016/j.marenvres.2008.09.006 |
Li H, Liu J G, Zhang L T, Pang T. 2016a. Effects of low temperature stress on the antioxidant system and photosynthetic apparatus of Kappaphycus alvarezii (Rhodophyta, Solieriaceae). Marine Biology Research, 12(10): 1064-1077.
DOI:10.1080/17451000.2016.1232827 |
Li H, Liu J G, Zhang L T, Pang T. 2016b. Antioxidant responses and photosynthetic behaviors of Kappaphycus alvarezii and Kappaphycus striatum (Rhodophyta, Solieriaceae) during low temperature stress. Botanical Studies, 57: 21.
DOI:10.1186/s40529-016-0136-8 |
Li Y F, Liu J G, Zhang L T, Pang T, Qin R Y. 2019. Effects of temperature on the photosynthetic performance in mature thalli of the red alga Gelidium amansii (Gelidiaceae). Aquaculture, 512: 734320.
DOI:10.1016/j.aquaculture.2019.734320 |
Li Y F, Liu J G, Zhang L T, Pang T. 2016c. Changes of photosynthetic performances in mature thalli of the red alga Gelidium amansii (Gelidiaceae) exposed to different salinities. Marine Biology Research, 12(6): 631-639.
DOI:10.1080/17451000.2016.1177654 |
Mathur S, Agrawal D, Jajoo A. 2014. Photosynthesis: response to high temperature stress. Journal of Photochemistry and Photobiology B: Biology, 137: 116-126.
|
Mathur S, Allakhverdiev S I, Jajoo A. 2011a. Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of photosystem II in wheat leaves (Triticum aestivum). Biochimica et Biophysica Acta, 1807(1): 22-29.
DOI:10.1016/j.bbabio.2010.09.001 |
Mathur S, Jajoo A, Mehta P, Bharti S. 2011b. Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biology, 13(1): 1-6.
DOI:10.1111/j.1438-8677.2009.00319.x |
Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7(9): 405-410.
DOI:10.1016/S1360-1385(02)02312-9 |
Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T. 2002. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell, 110(3): 361-371.
DOI:10.1016/S0092-8674(02)00867-X |
Muraoka R, Okuda K, Kobayashi Y, Shikanai T. 2006. A eukaryotic factor required for accumulation of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Physiology, 142(4): 1683-1689.
DOI:10.1104/pp.106.088682 |
Naeem M, Traub J R, Athar H U R, Loescher W. 2020. Exogenous calcium mitigates heat stress effects in common bean: a coordinated impact of photoprotection of PSII, up-regulating antioxidants, and carbohydrate metabolism. Acta Physiologiae Plantarum, 42(12): 180.
DOI:10.1007/s11738-020-03171-4 |
Niu J F, Feng J H, Xie X J, Gao S, Wang G C. 2016. Involvement of cyclic electron flow in irradiance stress responding and its potential regulation of the mechanisms in Pyropia yezoensis. Chinese Journal of Oceanology and Limnology, 34(4): 730-739.
DOI:10.1007/s00343-016-4236-9 |
Price G D. 2011. Inorganic carbon transporters of the cyanobacterial CO2 concentrating mechanism. Photosynthesis Research, 109(1-3): 47-57.
DOI:10.1007/s11120-010-9608-y |
Sampath-Wiley P, Neefus C D, Jahnke L S. 2008. Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: fluctuations in antioxidant contents, photosynthetic pigments and photosynthetic efficiency in the red alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). Journal of Experimental Marine Biology and Ecology, 361(2): 83-91.
DOI:10.1016/j.jembe.2008.05.001 |
Sharkey T D, Schrader S M. 2006. High temperature stress. In: Rao K V M, Raghavendra A S, Reddy K J eds. Physiology and Molecular Biology of Stress Tolerance in Plants. Springer, Dordrecht. p. 101-129.
|
Shikanai T. 2007. Cyclic electron transport around photosystem I: genetic approaches. Annual Review of Plant Biology, 58: 199-217.
DOI:10.1146/annurev.arplant.58.091406.110525 |
Shikanai T. 2016. Regulatory network of proton motive force: contribution of cyclic electron transport around photosystem I. Photosynthesis Research, 129(3): 253-260.
DOI:10.1007/s11120-016-0227-0 |
Takahashi S, Milward S E, Fan D Y, Chow W S, Badger M R. 2009. How does cyclic electron flow alleviate photoinhibition in Arabidopsis?. Plant Physiology, 149(3): 1560-1567.
DOI:10.1104/pp.108.134122 |
Teng F, Fang G H, Xu X Q. 2017. Effects of internal tidal dissipation and self-attraction and loading on semidiurnal tides in the Bohai Sea, Yellow Sea and East China Sea: a numerical study. Chinese Journal of Oceanology and Limnology, 35(5): 987-1001.
DOI:10.1007/s00343-017-6087-4 |
Várkonyi Z, Bajkán S, Váradi G, Lehoczki E. 2005. Light response of the chlorophyll fluorescence parameters and partitioning of absorbed light energy in wild type and npq4 mutant of Arabidopsis thaliana. Acta Biologica Szegediensis, 49(1-2): 229-232.
|
Wang C J, Yamamoto H, Shikanai T. 2015. Role of cyclic electron transport around photosystem I in regulating proton motive force. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1847(9): 931-938.
DOI:10.1016/j.bbabio.2014.11.013 |
Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye J Y, Mi H L. 2006. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiology, 141(2): 465-474.
DOI:10.1104/pp.105.070490 |
Wang W X, Vinocur B, Shoseyov O, Altman A. 2004. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends in Plant Science, 9(5): 244-252.
DOI:10.1016/j.tplants.2004.03.006 |
Xie E Y, Sun Q H. 2018. Cultivation of Gelidium. In: He P M, Zhang Z Y, Zhang X C, Ma J H eds. Seaweed Cultivation. Science Press, Beijing. p. 355-380. (in Chinese)
|
Yamori W, Shikanai T. 2016. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annual Review of Plant Biology, 67: 81-106.
DOI:10.1146/annurev-arplant-043015-112002 |
Yang X L, Xu H, Li T L, Wang R. 2017. Effects of exogenous melatonin on photosynthesis of tomato leaves under drought stress. Scientia Agricultura Sinica, 50(16): 3186-3195.
(in Chinese with English abstract) |
Yang Y F, Chai Z Y, Wang Q, Chen W Z, He Z L, Jiang S J. 2015. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Research, 9: 236-244.
DOI:10.1016/j.algal.2015.03.017 |
Yin Y, Li S M, Liao W Q, Lu Q T, Wen X G, Lu C M. 2010. Photosystem II photochemistry, photoinhibition, and the xanthophyll cycle in heat-stressed rice leaves. Journal of Plant Physiology, 167(12): 959-966.
DOI:10.1016/j.jplph.2009.12.021 |
Zaneveld J S. 1969. Factors controlling the delimitation of littoral benthic marine algal zonation. American Zoologist, 9(2): 367-391.
DOI:10.1093/icb/9.2.367 |
Zhang L T, He M L, Liu J G, Li L. 2015. Role of the mitochondrial alternative oxidase pathway in hydrogen photoproduction in Chlorella protothecoides. Planta, 241(4): 1005-1014.
DOI:10.1007/s00425-014-2231-y |
Zhang L T, Liu J G. 2016. Effects of heat stress on photosynthetic electron transport in a marine cyanobacterium Arthrospira sp. Journal of Applied Phycology, 28(2): 757-763.
|
Zhang L T, Su F, Zhang C H, Gong F Y, Liu J G. 2017. Changes of photosynthetic behaviors and photoprotection during cell transformation and astaxanthin accumulation in Haematococcus pluvialis grown outdoors in tubular photobioreactors. International Journal of Molecular Sciences, 18(1): 33.
|
Zhang L T, Zhang Z S, Gao H Y, Xue Z C, Yang C, Meng X L, Meng Q W. 2011. Mitochondrial alternative oxidase pathway protects plants against photoinhibition by alleviating inhibition of the repair of photodamaged PSII through preventing formation of reactive oxygen species in Rumex K-1 leaves. Physiologia Plantarum, 143(4): 396-407.
DOI:10.1111/j.1399-3054.2011.01514.x |
 2022, Vol. 40
2022, Vol. 40


