Institute of Oceanology, Chinese Academy of Sciences
Article Information
- GAO Fei, ZHANG Yue, WU Peilin, CHEN Mengling, HE Linwen, XU Qiang, WANG Aimin
- Bacterial community composition in gut content and ambient sediment of two tropical wild sea cucumbers (Holothuria atra and H. leucospilota)
- Journal of Oceanology and Limnology, 40(1): 360-372
- http://dx.doi.org/10.1007/s00343-021-1001-5
Article History
- Received Jan. 3, 2021
- accepted in principle Jan. 14, 2021
- accepted for publication Feb. 2, 2021
2 Ocean College, Hainan University, Haikou 570228, China
Sea cucumbers (Echinodermata: Holothuroidea) are important benthic marine invertebrate resources found in all of the world's major oceans and seas. Up to date, there are 1 750 accepted extant holothuroid species in the world (WoRMS, 2020). Of these, about 66 species of sea cucumber are commonly exploited in multiple locations (Purcell, 2010; Purcell et al., 2012; Conand, 2018). In Asia and the Middle East, some sea cucumbers are considered delicacies, a source of protein, folk medicine, or an important part of income (Bordbar et al., 2011; Purcell et al., 2012; Yang et al., 2015). As large and abundant members of marine benthic communities, their feeding and excretion have crucial effects on the physicochemical processes of soft-bottom and reef ecosystem. They can be the host of many species of symbionts and parasites, thus enhancing biodiversity; and they are preyed by many taxa, thereby transferring nutrients from detritus to higher trophic levels (MacTavish et al., 2012; Schneider et al., 2013; Purcell et al., 2016).
Bacteria are vital in coral reef processes, such as organic matter decomposition and nutrient recycling in reef food chains. Because of the high abundance, production, and nutritional values, the bacteria in the sediment are a direct food source or a source that indirectly provides the host with essential nutrients, such as B-complex vitamins for marine detritus feeders (Gerlach, 1978; Deming and Colwell, 1982; Moriarty, 1982; Phillips, 1984; Amaro et al., 2009). As typical common detritus feeders, sea cucumbers primarily digest bacteria, decaying seagrass and algae, diatoms, flagellate, protozoan, foraminiferans, fungi, and other organic matter, which constitute detritus (Yingst, 1976; Moriarty, 1982; Uthicke, 1999; Gao et al., 2010; MacTavish et al., 2012). In particular, bacteria have been a commonly-reported component of holothuroid diets (Moriarty, 1982; Gao et al., 2010; Wen et al., 2016). Feeding activity of sea cucumbers takes a strong part in the bioturbation of marine benthic ecosystems by removing considerable amounts of sediments during gut passage (Bonham and Held, 1963; Mangion et al., 2004), so we supposed sea cucumbers could affect the sediment bacterial community structure through their ingestion and digestion processes.
Sea cucumbers Holothuria leucospilota and H. atra are both exploited as edible sea cucumbers belonging to the genus Holothuria. These sea cucumbers naturally distribute in tropical seas. Their populations are relatively dense and shallow subtidal, commonly found on inner and outer reef flats, back reefs, and shallow coastal lagoons (Purcell et al., 2016; Conand, 2018). They are known as depositfeeders, gathering organic detritus and sediments from the sediment surface. Nevertheless, for these two sea cucumber species, the researches mainly focus on taxonomic identification (Wen et al., 2011), extraction of bioactive materials (Han et al., 2007; Ibrahim, 2012; Esmat et al., 2013; Kitisin et al., 2019; Darya et al., 2020; Zhao et al., 2020), breeding and rearing (Huang et al., 2018), and ecological effects on recycling nutrient in reefs (Bonham and Held, 1963; Vidal-Ramire and Dove, 2016; Viyakarn et al., 2020).
However, for H. leucospilota and H. atra, the gut bacterial community composition and their effects on sediment bacterial community by their feeding activity have been surveyed sporadically. The amounts of culturable aerobic bacterial flora in the digestive tract of H. atra and the surrounding sediment were investigated, and some isolates were identified by 16S ribosomal DNA (16S rDNA) sequence analysis (Ward-Rainey et al., 1996). Zhang et al. (2012) isolated and identified 141 bacterial strains under aerobic conditions from the gut of H. leucospilota, and detected the polysaccharide degradation activities by plate methods using carboxymethyl cellulose sodium salt, xylan, alginate, starch, or agar as the substrate. The amounts of culturable bacteria were measured in the substrate, anterior and posterior gut of H. scabra, H. atra, and H. leucospilota (Hatmanti and Purwati, 2011). Muramic acid, the component of bacterial cell wall peptidoglycan, is often used as an indicator of the content of bacteria in environmental samples. Moriarty (1982) determined the bacterial biomass by muramic acid measurements in the sediments and gut contents of H. atra on the Great Barrier Reef. The previous research mainly studied the gut microbiota, which could be cultured in sea cucumbers. As most of the bacteria in the nature are non-culturable under laboratory conditions, the characteristics of a tiny proportion of the total bacterial community in the gut of sea cucumbers were revealed. Thus, it is necessary to apply culture-independent methods to improve the understanding of total microbiota in the digestive tract and sea cucumbers' habitat. High-throughput sequencing techniques have been used to analyze the microbiota in various biomes, such as the gastrointestinal tracts of humans and animals, soils, water, and sediments (Warnecke et al., 2007; McLellan et al., 2010; Hess et al., 2011; Gao et al., 2014a; Jia et al., 2020).
In this study, we examined the bacterial community composition in the habitat surface sediment, the foregut and hindgut contents of two species of common tropical sea cucumbers H. atra and H. leucospilota by 16S rRNA gene high-throughput sequencing. The objectives of this study were: 1) characterize and compare the bacterial community diversity and abundance in gut contents of the two species of sea cucumbers; 2) compare the bacterial community structures ingested by sea cucumbers (in foregut) with the surrounding sediments so as to investigate the sea cucumbers' feeding selectivity concerning to sediment bacteria; 3) compare the bacterial community structures in the hindgut of sea cucumbers with the surrounding sediments in order to identify effects of the feeding of sea cucumbers on environmental microorganisms.
2 MATERIAL AND METHOD 2.1 MaterialSea cucumbers were collected from a natural distribution area locating in the intertidal zone of Tongguling natural reserve in Wenchang, Hainan Province, China. H. leucospilota (average wet weight of 392.10±71.89 g (n=5)) and H. atra (average wet weight of 131.84±11.98 g (n=5)) individuals were collected on April 20, 2019. Ambient surface sediments (0–1.2 cm, n=5) were collected accordingly from 5 locations around the sampled sea cucumbers using 50-mL syringe samplers (Michio et al., 2003; Gao et al., 2014a). The water temperature and salinity at the sampling site was 28.4 ℃ and 31. Then, the sea cucumber and sediment samples were stored on ice and transported to the laboratory immediately.
Under sterile conditions, sea cucumbers were washed with sterile seawater, and the scarfskin was sterilized with 75% ethanol to reduce exogenous bacterial contamination. The ventral body walls were incised using a sterile scalpel to expose the body cavity. During the process of dissection, we found the two species of sea cucumbers have long guts with the whole length of 83.40±8.52 cm and 43.30±3.43 cm in H. leucospilota and H. atra, respectively. In order to investigate the changes of the gut microbial community which were just ingested and the contents which were about to be discharged, the foregut (the most anterior 2–3-cm part of the gut) and hindgut contents (the most posterior 2–3-cm part of the gut) were separately collected in sterile freezing tubes (Gao et al., 2014a; Mfilinge and Tsuchiya, 2016). The foregut samples of H. leucospilota and H. atra were labeled as YQ1-YQ5 and HQ1-HQ5, respectively, and the hindgut samples were marked as YH1-YH5 and the HH1-HH5, respectively. The sediments were marked by DN1-DN5. All samples were stored at -80 ℃ for further analysis.
2.2 Method 2.2.1 DNA extraction and polymerase chain reaction (PCR) amplificationOmega Soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA) was used to extract DNA from the gut content and sediment according to the manufacturer's protocol.
PCR amplification of bacterial 16S rRNA gene hypervariable regions (V3-V4) was conducted using the universal primers, including 341F (5'-CCTAYGGGRBGCASCAG-3') and 806R (5'-GGACTACNNGGGTATCTAAT-3'), with barcodes to allow sample multiplexing (Wang et al., 2018; Gao et al., 2019). A total of 30-μL PCR mixture contained 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μmol/L of forward and reverse primers, and 10-ng template DNA. Thermal cycling consisted of initial denaturation at 98 ℃ for 1 min, followed by 30 cycles of denaturation at 98 ℃ for 10 s, annealing at 50 ℃ for 30 s, elongation at 72 ℃ for 30 s and a final extension step at 72 ℃ for 5 min. PCR products were mixed in equidensity ratios and purified with GeneJETTM Gel Extraction Kit (Thermo Scientific).
2.2.2 Library preparation and sequencingSequencing libraries were generated using Ion Plus Fragment Library Kit 48 rxns (Thermo Scientific) following the manufacturer's instructions. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific). The library was sequenced on an Ion S5TM XL platform, and 400-bp/600-bp singleend reads were generated.
2.2.3 Statistical and bioinformatics analysisThe data were analyzed using Statistical Package for the Social Sciences (SPSS) 19.0. All values were presented as the means±standard error (mean±SE). The level of statistical significance was determined using the t-test. The statistical significance was set at P < 0.05.
Single-end reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequences. Quality filtering on the raw reads was performed under specific filtering conditions according to the Cutadapt (V1.9.1, http://cutadapt.readthedocs.io/en/stable/) quality-controlled process (Martin, 2011). The reads were compared with the reference database (Silva database, https://www.arb-silva.de/) using UCHIME algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html) to detect chimera sequences, and the chimera sequences were then removed (Edgar et al., 2011).
Sequences analysis was performed by Uparse software (Uparse v7.0.1001, http://drive5.com/uparse/; Edgar, 2013). The sequences with ≥97% similarity were assigned to the same operational taxonomic units (OTUs). The representative sequence for each OTU was screened for further annotation. For each representative sequence, the Silva Database (https://www.arb-silva.de/) was used based on the Mothur algorithm to annotate taxonomic information.
Alpha diversity is applied in analyzing complexity of species diversity for a sample through 4 indices, including the abundance-based coverage estimator (ACE), bias-corrected Chao1 richness estimator, and the Shannon and Simpson diversity indices. were calculated. All the analyses were performed using the Mothur program (http://www.mothur.org). The microbial community structures in different samples were calculated by Quantitative Insights Into Microbial Ecology (QIIME2) software (version 1.7.0). Non-metric multidimensional scaling (NMDS) analysis was performed with the R software (Version 2.15.3). Unweighted Pair-group Method with Arithmetic Means (UPGMA) Clustering was performed using average linkage and conducted by QIIME2 software (Version 1.7.0).
3 RESULTA total of 2 002 434 optimized reads with an average length of 408 bp were obtained from all the samples. The optimized read numbers for each sample ranged from 80 023 to 80 174, with a mean average of 80 097±9. The Good's coverage of all samples was between 98.9% and 99.5%, which showed that the sequencing depth was adequate to cover most microorganisms.
3.1 Richness and diversityA total of 5 584 OTUs were obtained from 25 samples based on a 97% threshold. The foregut content samples from each of the five H. leucospilota (YQ1–YQ5) contained 1 145–1 592 OTUs, and the hindgut contents (YH1–YH5) from each of the five sea cucumbers contained 940 to 1 427 OTUs. The foregut (HQ1–HQ5) and hindgut contents (HH1–HH5) from each of the five H. atra contained 1 016–2 134 OTUs, and 1 101–1 411 OTUs, respectively. The five surface sediments (DN1–DN5) contained 2 497–2 635 OTUs.
Of these OTUs, 369, 147, 109, 177, and 1 088 OUT were detected in YQ, YH, HQ, HH, and DN, respectively (Fig. 1). A total of 995 OTUs were shared by all the gut content samples and their surrounding sediments (Fig. 1).
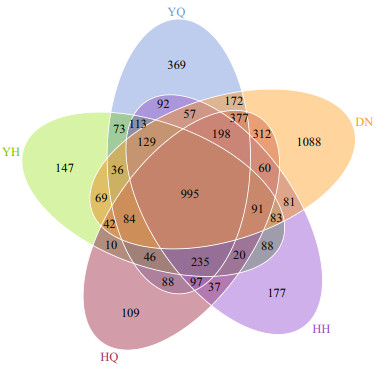
|
| Fig.1 Venn diagram of core OTUs among the foregut (YQ), hindgut (YH) of H. leucospilota, the foregut (HQ), hindgut (HH) of H. atra and the surrounding sediments (DN) |
The bacterial community richness was calculated by estimating the number of OTUs based on the ACE and Chao1 values at the 3% dissimilarity level. The ACE and Chao1 values in the sediment samples were significantly higher than the values in all the foregut and hindgut contents of two species of sea cucumbers (Fig. 2a & b; P < 0.05), indicating that the bacterial richness in the sediment samples was higher than that in the gut content samples. However, there were no significant differences while comparing between the same parts of the guts of the two sea cucumber species (P > 0.05).

|
| Fig.2 Alpha-diversity of microbial communities in the foregut (YQ), hindgut (YH) of H. leucospilota, the foregut (HQ), hindgut (HH) of H. atra and the surrounding sediments (DN): ACE (a), Chao1 (b), Shannon index (c), and Simpson index (d) *: P < 0.05; **: P < 0.01. Dark point represents the abnormal value. |
The Shannon and Simpson diversity indices in the sediment samples were higher than the indices in all the gut content samples (Fig. 2c & d; P < 0.05), but there were no significant differences while comparing between the same parts of gut of the two sea cucumber species (P > 0.05). The results suggested that the bacterial diversity in the habitat sediments was higher than that in the gut contents of the sea cucumbers, but not clear difference between the gut contents of the two sea cucumbers (P > 0.05).
3.2 Bacterial community structure in gut contents and sedimentsMost of the reads (97.6%–99.8%) could be classified at the phylum level in all samples. Of these, a total of 39 and 31 different phyla were identified in the foregut and hindgut contents of H. leucospilota. In the foregut and hindgut contents of H. atra, the bacteria could be separately classified into 36 and 35 different phyla. The identified bacteria community in the ambient sediments was 40 phyla.
The ten most abundant phyla of the different samples are listed in Fig. 3. Proteobacteria was the predominant phylum, showing 45.69%±8.61%, 70.09%±4.03%, 45.88%±5.38%, and 55.19%±0.79% reads in the YQ, YH, HH, and DN libraries, respectively. In the HQ samples, the relative content of Proteobacteria was 20.33%±5.75%, while Bacteroidetes was the predominant phylum with a relative content of 34.98%±5.52%. There was no significant difference in the abundance of Proteobacteria among the foregut and hindgut contents in H. leucospilota and sediment samples (P > 0.05). The abundance of Proteobacteria in the hindgut content of H. atra was significantly lower than that in surrounding sediments and foregut content (P < 0.05).
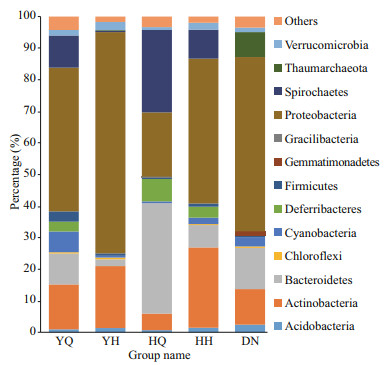
|
| Fig.3 The relative abundance of the 10 most abundant phyla YQ and YH respectively represent the foregut and hindgut of sea cucumber H. leucospilota. HQ and HH respectively represent the foregut and hindgut of sea cucumber H. atra. DN represents the surrounding sediments. |
While comparing the bacterial abundance in foregut contents with that in sediments at the phylum level, the contents of Thaumarchaeota, Acidobacteria, Gemmatimonadetes, Latescibacteria, Fusobacteria, Nitrospirae, and Calditrichaeota in the foregut of H. leucospilota were significantly lower than those in sediments (P < 0.05; Fig. 4a). The contents of Proteobacteria, Thaumarchaeota, Acidobacteria, Gemmatimonadetes, Latescibacteria, Fusobacteria, Nitrospirae, and Calditrichaeota in the foregut of H. atra were significantly lower than those in sediments (P < 0.05), but the contents of Bacteroidetes and Spirochaetes in the foregut of H. atra were significantly higher than those in sediments (P < 0.05; Fig. 4b). The relative abundance of Bacteroidetes, Thaumarchaeota, Acidobacteria, Gemmatimonadetes, Latescibacteria, Fusobacteria, Nitrospirae, and Calditrichaeota in the hindgut of H. leucospilota were significantly lower than those in sediments, but the contents of Proteobacteria and Verrucomicrobia in the hindgut of H. leucospilota were significantly higher than those in sediments (P < 0.05; Fig. 4c). The contents of Thaumarchaeota, Acidobacteria, Gemmatimonadetes, Chloroflexi, Planctomycetes, Latescibacteria, Fusobacteria, Nitrospirae, and Calditrichaeota in the hindgut of H. atra were significantly lower than those in sediments (P < 0.05), but the contents of Actinobacteria and Dadabacteria in the hindgut of H. atra were significantly higher than those in sediments (P < 0.05; Fig. 4d).

|
| Fig.4 Identified differentially abundant phyla between YQ and DN (a), HQ and DN (b), YH and DN (c), HH and DN (d), YQ and HQ (e), YH and HH (f) (P < 0.05) YQ and YH represent the foregut and hindgut of sea cucumber H. leucospilota, respectively. HQ and HH represent the foregut and hindgut of sea cucumber H. atra, respectively. DN represents the surrounding sediments. The dotted line represents that the difference of means is equal to 0. |
Most of the taxa had no significant differences while comparing the bacterial abundance in the same part between the two sea cucumber species. The content of Proteobacteria in the foregut of H. leucospilota was significantly higher than that in the foregut of H. atra (P < 0.05), and Bacteroidetes content in the foregut of H. leucospilota was significantly lower than that in foregut of H. atra (P < 0.05; Fig. 4e). As for the hindgut, only the content of Proteobacteria in H. leucospilota was significantly higher than that in H. atra (P < 0.05; Fig. 4f).
At the genus level, a large proportion (21.5%–83.2%) of the reads could not be classified. The ten most abundant genera in the different samples accounted for 35.39%, 49.73%, 29.09%, 45.01%, and 20.51% reads in the YQ, YQ, HQ, HH, and SD libraries, respectively (Fig. 5). The most abundant genera in the foregut content libraries of H. leucospilota were sequences related to Aeromicrobium (8.06%±7.34%), Ruegeria (7.40%±2.85%), unidentified Cyanobacteria (5.83%±3.27%), Ilumatobacter (2.97%±0.82%), Sulfurospirillum (2.25%±1.37%), Halioglobus (2.10%±1.03%), Anderseniella(1.91%±1.08%), Vibrio(1.84%±1.34%), Dinoroseobacter (1.63%±0.67%), and unidentified Spirochaetaceae (1.40%±0.88%). In the hindgut libraries of H. leucospilota, the most abundant genera (relative abundance > 1%) were Ruegeria (24.87%±6.25%), Ilumatobacter (8.91%±3.35%), Aeromicrobium (6.00%±2.47%), Dinoroseobacter (4.03%±1.22%), Acinetobacter (1.36%±1.21%), Woeseia (1.27%±0.37%), Anderseniella (1.13%±0.28%), and Halioglobus (1.01%±0.18%). The most abundant genera in the foregut content libraries of H. atra were sequences related to unidentified Spirochaetaceae (20.99%±5.80%), Woeseia (4.03%±1.22%), Ruegeria (1.35%±0.67%), Ilumatobacter (4.03±1.22%), and Sulfurospirillum (1.01±0.42%). In the hindgut libraries of H. atra, the most abundant genera (relative abundance > 1%) were Ruegeria (10.36%±5.05%), Aeromicrobium (9.75%±4.46%), Ilumatobacter (9.57%±1.27%), unidentified Spirochaetaceae (6.77%±4.23%), Anderseniella (2.20%±0.32%), Dinoroseobacter (2.14%±0.49%), Woeseia (1.38%±0.41%), and Cohaesibacter (1.09±0.19%). In the sediment libraries, Woeseia (7.06%±0.97%), Ilumatobacter (2.53%±0.32%), unidentified Cyanobacteria (2.50%±0.79%), unidentified Gammaproteobacteria (2.14%±0.15%), unidentified Acidobacteria (1.62%±0.18%), unidentified Alphaproteobacteria (1.09%±0.44%) and Anderseniella (1.01%±0.10%) were the dominant genera (relative abundance > 1%).
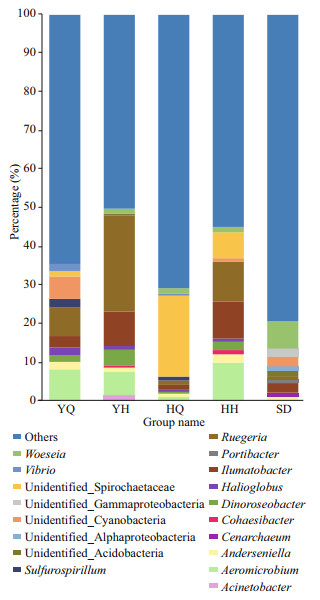
|
| Fig.5 The relative abundance of the 10 most abundant genera YQ and YH represent the foregut and hindgut of sea cucumber H. leucospilota, respectively. HQ and HH represent the foregut and hindgut of sea cucumber H. atra, respectively. DN represents the surrounding sediments. |
Among the dominant genera, reads related to the genera Anderseniella, Ilumatobacter, and Ruegeria were detected in all the gut contents and sediment libraries. In the hindgut of the two species of sea cucumber, the abundance of genus Ruegeria was very high, 24.87%±6.25% and 10.36%±5.05% in H. leucospilota and H. atra, respectively, and the Ruegeria abundance in the hindgut of H. leucospilota was significantly higher than that in the other samples (P < 0.05).
3.3 Relationships among the bacterial communities from the gut content and the sediment samplesNMDS analysis and UPGMA tree were performed to assess the relatedness of the microbial community composition among different samples (Figs. 6 & 7). The analyses indicated that gut microbiota in the foregut and hindgut of H. atra and sediment were clustered separately into three groups, which showed that, for H. atra, the foregut, hindgut, and sediment had obviously different characteristics of bacterial communities. The NMDS analysis showed that the bacterial community composition of the foregut in H. leucospilotawas overlapped with the characteristics of the hindgut samples of two sea cucumber species and the foregut samples of H. atra. The bacterial community of sediment was different from all the foregut and hindgut bacterial communities in both H. atra and H. leucospilota. The hindguts of H. atra and H. leucospilota had comparatively similar bacterial communities.

|
| Fig.6 Nonmetric multidimensional scaling (NMDS) plot based on Bray-Curtis distance showing the relatedness of the bacterial community composition between the different samples YQ and YH represent the foregut and hindgut of sea cucumber H. leucospilota, respectively. HQ and HH represent the foregut and hindgut of sea cucumber H. atra, respectively. DN represents the surrounding sediments. |
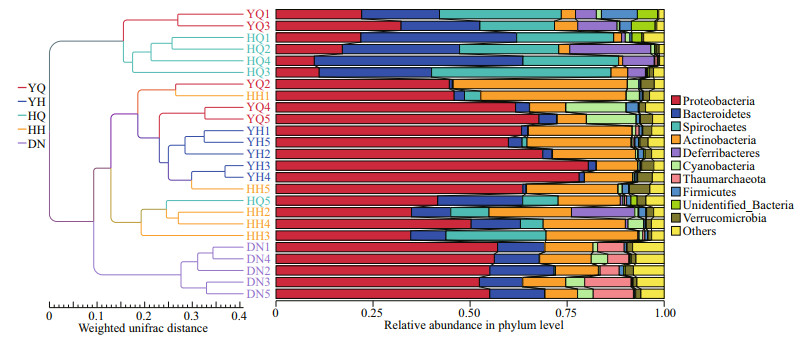
|
| Fig.7 UPGMA tree showing the similarity of bacterial community structures among the different samples YQ and YH represent the foregut and hindgut of sea cucumber H. leucospilota, respectively. HQ and HH represent the foregut and hindgut of sea cucumber H. atra, respectively. DN represents the surrounding sediments. |
As typical deposit-feeding or detritus-feeding marine invertebrates, the feeding and nutrition of aspidochirotid sea cucumbers have received considerable attention. In this study, we investigated the bacterial community composition in different parts of gut of two commercially exploited tropical sea cucumbers species H. atra and H. leucospilota and in their surrounding sediments, in order to assess the sea cucumber's feeding strategy and the effects on the sedimentary microbiota.
4.1 Dominant bacteria in the gut contentsA total of 46 different phyla were identified from the gut contents of two species of sea cucumbers, and their ambient sediments showed 40 phyla. Of these, Proteobacteria was the predominant phylum in all the gut content samples of H. leucospilota, hindgut contents of H. atra and sediments. In previous studies, Proteobacteria was found to be the predominant phylum in the gut contents of Apostichopus japonicus (Gao et al., 2014a; Wang et al., 2018), marine water, and sediments (Paliaga et al., 2017; Wang et al., 2019; Custodio et al., 2020).
Ruegeria was the predominant bacteria in the hindgut content of two species of sea cucumbers in our study, and the content in the hindgut of H. leucospilota was higher than that in the other samples (P < 0.05). The genus Ruegeria was first reported by Uchino et al. (1998) based on the reclassification of Agrobacterium gelatinovorum and Roseobacter algicola as Ruegeria gelatinovora and R. algicola, respectively. Till now, the genus Ruegeria comprises about 18 species which have been named, and most of these are isolated from marine environments, including marine sediment, seawater, seashore, sea squirt, and oyster (Arahal et al., 2018; Kim et al., 2019; Baek et al., 2020). The common characteristics of the genus Ruegeria are nonsporulating, non-motile, ovoid, or rod-shaped cells that contain Q-10 as a predominant respiratory quinone (Baek et al., 2020). Species of the genus are chemoorganotrophic aerobes, slightly halophilic and mesophilic, and none of them can synthesize bacteriochlorophyll a (Arahal et al., 2018). The content of Woeseia in sediments was significantly higher than that of two species of sea cucumbers (P < 0.001). The Woeseia bacteria are Gram-negative, rods or bent rods, non-motile, and facultatively anaerobic, and require salt to grow. Woeseia showed an unusually high abundance in marine sediments, including coastal sediments, due to the broad range of energy-yielding metabolisms (Muβmann et al., 2017).
Vibrio has been found widely distributed in the digestive tract of sea cucumbers, such as A. japonicus (Sun and Chen, 1989; Gao et al., 2014a), H. atra (Ward-Rainey et al., 1996), and H. leucospilota (Zhang et al., 2012). In the present study, Vibrio was found in all the gut contents, sediment samples, and foreguts of two sea cucumbers species. Although Vibrio isolates are commonly believed to be pathogenic for aquatic animals, a lot of avirulent strains have been used as probiotics (Rahiman et al., 2010; Silva-Aciares et al., 2011; Pandiyan et al., 2013). Vibrio sp. V33 isolated from healthy sepia showed intense antagonistic activity against pathogenic Vibrio splendidus Vs (Liu et al., 2017). A total of 13 species of Vibrio isolated from the digestive tract of H. leucospilota showed amylase and alginatelyase activities (Zhang et al., 2012). Chi et al. (2014) isolated V. tasmaniensis from the gut of A. japonicus and considered this strain as potential probiotics in A. japonicus after demonstrating its extra-cellular amylase, lipase, and protease production capacities and a series of dietary supplementation tests.
4.2 Comparison of gut microbe composition in H. leucospilota and H. atraWhile comparing the bacterial abundance in the same gut part between the two species of sea cucumbers, most of the taxa had no significant variations. In the foregut contents, only Proteobacteria and Bacteroidetes had significantly different ranges between H. leucospilota and H. atra. NMDS analysis showed the foregut content samples of H. atra grouped intensively, yet the bacterial community composition of the foregut in H. leucospilota was overlapped with the characteristics of the hindgut samples of two sea cucumber species and the foregut samples of H. atra, which indicated microbial food sources of sea cucumber H. leucospilota has much more differences among individuals than H. atra. In other words, H. atra might have a stronger feeding preference than H. leucospilota.
In the hindgut contents, H. atra and H. leucospilota had comparatively similar bacterial communities, with only the content of Proteobacteria in H. leucospilota differing significantly from H. atra. Similar gut sediment fatty acid composition and concentration, including fatty acid biomarkers for bacteria, were found in H. atra and H. leucospilota, and the authors considered that ingestion of the same type of organic material was the primary reason (Mfilinge and Tsuchiya, 2016). In this study, we speculated that the high similarity of bacterial community composition in hindgut contents between two sea cucumbers species was probably due to escaping digestion of the same type of microbes.
4.3 Relationship between bacterial communities in the foregut and the sedimentsIt has long been focused on whether deposit-feeding sea cucumbers could feed selectively. Some reports have claimed that sea cucumbers feed selectively, particularly with respect to particle size (Hauksson, 1979; Roberts, 1979; Zhao, 2010; Xue et al., 2019), bacterial biomass, and community composition (Moriarty, 1982; Sun and Chen, 1989; Gao et al., 2014a, b), and organic matter content of sediments (Moriarty, 1982; Paltzat et al., 2008; Zhao, 2010; Xue et al., 2019). In most studies, it is usual to compare specific parameters of the foregut content with the corresponding parameters in the ambient sediment to describe the selectivity in sediment feeders.
Beside of selective feeding, microflora attaching to the gut wall or microbial reproduction in the gut might also be the possible sources of the distinct bacterial community composition in the adjacent sediments and foregut contents of sea cucumbers. In this study, we tried to minimize the two impact factors while extracting the samples. Firstly, merely the contents in the most anterior part of the foregut (about 2–3 cm, though the whole gut length in H. leucospilota and H. atra was 43.30±3.43 cm and 83.40±8.52 cm, respectively) were extracted as the foregut contents in order to reduce the effect of specific microbial reproduction in guts. We considered that there would not be much time for bacteria to grow there (Moriarty, 1982; Gao et al., 2014a). Secondly, the gut contents were squeezed out carefully, making certain that contents adhering to gut issue were excluded, so as to lessen the possible autochthonous bacteria sources. In this study, the bacterial community composition between the foregut content of two species of sea cucumbers and the surrounding sediments was compared, which indicated different bacterial composition between the sediment and foregut samples in H. leucospilota and H. atra. Therefore, we speculate that the different bacterial communities in the foregut content and sediments may mainly result from selective feeding. Moriarty (1982) found that the bacterial biomass and organic carbon levels in the foregut of H. atra were two to three-fold greater than in the surrounding sediment, and indicated that the animals selected sediment fractions that contained more bacteria and organic matters. We also investigated the sediment characteristics ingested by H. atra in a typical tropical coral reef in the South China Sea, and the results showed that the organic matter content and grain size ratio in the digestive tract of H. atra were significantly different from those in environmental sediments (Xue et al., 2019). Fatty acids, including saturated, monounsaturated, polyunsaturated, and branched fatty acids, were significantly higher in the foregut of H. atra and H. leucospilota than those in ambient sediments, suggesting that both species selectively ingested nutrient-rich particles (Mfilinge and Tsuchiya, 2016). As it is impossible to identify the microbe community diversity or abundance in diet for any species of animals, we supposed that the clearly different bacterial composition between foregut contents and the surrounding sediments might be due to their selective feeding of sediment patches.
Highly selective feeding has been observed in various species of sea cucumbers from temperate to tropical habitats, regardless of shallow-water ones or deep-sea ones (Khripounoff and Sibuet, 1980; Sloan and von Bodungen, 1980; Hammond, 1983; Billett et al., 1988; Amaro et al., 2009; Gao et al., 2014b), and the objects selected including grain size of particles, organic content, sediment patches, and so on. Another question is how the deposit-feeding sea cucumbers selectively feed. Holothuroids take in food by means of their oral tentacles. Histological examination indicated that the tentacle structure, including the size of nodules or nodule groups, inter-papillar spaces, and mucous secretion ability of the nodules were responsible for the physical selection for certain particle sizes (Fankboner, 1978; Roberts, 1979; Foster and Hodgson, 1996). As for the mechanisms of selective feeding of other parameters, such as organic contents and composition in sediments, it is theoretically required well developed sensory organs for sediment quality judging. Foster and Hodgson (1996) found that, in several species of sea cucumbers, the nodules at the terminal of each tentacle have lengthened epithelial cells with papillae comprising cilia, which are distinct from other tentacle parts. The papillae have been regarded as sensory receptors for feeding (Fankboner, 1978; Smith, 1983).
4.4 Impact on sediment bacterial community by feeding of sea cucumbersBacteria play an important role in coral reef processes consisting of organic matter decomposition, nutrient recycling, and so on. Sea cucumbers are prominent deposit-feeders as they can rework considerable quantity of sediments (Bonham and Held, 1963; Mangion et al., 2004), and it has long been proved that bacteria were one kind of important food sources for sea cucumbers (Moriarty, 1982; Uthicke, 1999; Gao et al., 2010; MacTavish et al., 2012). Therefore, sea cucumber's feeding activity may influence the bacterial community diversity and biomass in sediments. As it is difficult to find and collect the fresh feces in situ, we collected the posterior 2–3 cm of the gut contents as the hindgut samples, and we assumed that this section of gut content had the most similarity with the feces which would be passed out. In the present study, NMDS analysis and UPGMA tree both showed the bacterial community structure in hindgut content of the two species of sea cucumbers differed clearly from sediments. In details, the relative contents of ten phyla of bacteria including Proteobacteria, Bacteroidetes, Thaumarchaeota, Acidobacteria, Gemmatimonadetes, Latescibacteria, Fusobacteria, Nitrospirae, Calditrichaeota, and Verrucomicrobia in the hindgut of H. leucospilota were significantly different from those in sediments, and the relative contents of phyla Actinobacteria, Thaumarchaeota, Acidobacteria, Gemmatimonadetes, Chloroflexi, Planctomycetes, Latescibacteria, Fusobacteria, Nitrospirae, Dadabacteria, and Calditrichaeota in the hindgut of H. atra were significantly different from those in sediments. The results indicated that marked changes in sediment bacterial composition occurred during gut passage of the sediment particles in both sea cucumber species. Clearly different bacterial biomass between the hindgut contents of H. atra and sediments were also observed in Heron Island, Great Barrier Reef (Moriarty, 1982). When sediment bacterial biomass is low, feeding activity of H. tubulosa results in a net increase in bacteria during the gut passage, while comparatively high bacterial biomass in sediments leads to a net bacterial removal (Amon and Herndl, 1991). In conclusion, to some extent, depositfeeding sea cucumbers, including H. leucospilota and H. atra, could actually influence sediment bacterial community structure through their feeding activities.
5 DATA AVAILABILITY STATEMENTThe datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.
Amaro T, Witte H, Herndl G J, Cunha M R, Billett D S M. 2009. Deep-sea bacterial communities in sediments and guts of deposit-feeding holothurians in Portuguese canyons (NE Atlantic). Deep Sea Research Part Ⅰ Océanographie Research Papers, 56(10): 1 834-1 843.
DOI:10.1016/j.dsr.2009.05.014 |
Amon R M W, Herndl G J. 1991. Deposit feeding and sediment: Ⅰ. interrelationship between Holothuria tubulosa (Holothurioida, Ech'modermata) and the sediment microbial community. Marine Ecology, 12(2): 163-174.
DOI:10.1111/j.1439-0485.1991.tb00250.x |
Arahal D R, Lucena T, Rodrigo-Torres L, Pujalte M J. 2018. Ruegeria denitrificans sp. nov., a marine bacterium in the family rhodobacteraceae with the potential ability for cyanophycin synthesis. International Journal of Systematic and Evolutionary Microbiology, 68(8): 2 515-2 522.
DOI:10.1099/ijsem.0.002867 |
Baek J, Kim J H, Sukhoom A, Kim W. 2020. Ruegeria sediminis sp. nov., isolated from tidal flat sediment. International Journal of Systematic and Evolutionary Microbiology, 70(5): 3 055-3 061.
DOI:10.1099/ijsem.0.004128 |
Billett D S M, Llewellyn D, Watson J. 1988. Are deep-sea holothurians selective feeders? In: Burke R D, Mladenov P V, Lambert P, Parsley R L eds. Echinoderm Biology: Proceedings of the 6th International Echinoderm Conference. Balkema, Rotterdam. p. 421-429.
|
Bonham K, Held E E. 1963. Ecological observations on the sea cucumbers Holothuria atra and H. leucospilota at Rongelap Atoll, Marshall Islands. Pacific Science, 17(3): 305-314.
|
Bordbar S, Anwar F, Saari N. 2011. High-value components and bioactives from sea cucumbers for functional foods-A Review. Marine Drugs, 9(10): 1 761-1 805.
DOI:10.3390/md9101761 |
Chi C, Liu J Y, Fei S Z, Zhang C, Chang Y Q, Liu X L, Wang G X. 2014. Effect of intestinal autochthonous probiotics isolated from the gut of sea cucumber (Apostichopus japonicus) on immune response and growth of A. japonicus. Fish & Shellfish Immunology, 38(2): 367-373.
|
Conand C. 2018. Tropical sea cucumber fisheries: changes during the last decade. Marine Pollution Bulletin, 133: 590-594.
DOI:10.1016/j.marpolbul.2018.05.014 |
Custodio M, Ordinola-Zapata A, Espinoza C, Vieyra-Peña E, Peñaloza R, Sánchez-Suárez H, Peralta-Ortiz T. 2020. Metagenomic data on the composition of bacterial communities in lake environment sediments for fish farming by next generation Illumina sequencing. Data in Brief, 32: 106228.
DOI:10.1016/j.dib.2020.106228 |
Darya M, Sajjadi M M, Yousefzadi M, Sourinejad I, Zarei M. 2020. Antifouling and antibacterial activities of bioactive extracts from different organs of the sea cucumber Holothuria leucospilota. Helgoland Marine Research, 74(1): 4.
DOI:10.1186/s10152-020-0536-8 |
Deming J W, Colwell R R. 1982. Barophilic bacteria associated with digestive tracts of Abyssal Holothurians. Applied and Environmental Microbiology, 44(5): 1 222-1 230.
DOI:10.1128/aem.44.5.1222-1230.1982 |
Edgar R C, Haas B J, Clemente J C, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 27(16): 2 194-2 200.
DOI:10.1093/bioinformatics/btr381 |
Edgar R C. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 10(10): 996-998.
DOI:10.1038/nmeth.2604 |
Esmat A Y, Said M M, Soliman A A, El-Masry K S H, Badiea E A. 2013. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition, 29(1): 258-267.
DOI:10.1016/j.nut.2012.06.004 |
Fankboner P V. 1978. Suspension-feeding mechanisms of the armoured sea cucumber Psolus chitinoides Clark. Journal of Experimental Marine Biology and Ecology, 31(1): 11-25.
DOI:10.1016/0022-0981(78)90133-8 |
Foster G G, Hodgson A N. 1996. Feeding, tentacle and gut morphology in five species of southern African intertidal holothuroids (Echinodermata). South African Journal of Zoology, 31(2): 70-79.
DOI:10.1080/02541858.1996.11448396 |
Gao F, Li F H, Tan J, Yan J P, Sun H L. 2014a. Bacterial community composition in the gut content and ambient sediment of sea cucumber Apostichopus japonicus revealed by 16S rRNA gene pyrosequencing. PLoS One, 9(6): e100092.
DOI:10.1371/journal.pone.0100092 |
Gao F, Tan J, Sun H L, Yan J P. 2014b. Bacterial diversity of gut content in sea cucumber (Apostichopus japonicus) and its habitat surface sediment. Journal of Ocean University of China, 13(2): 303-310.
DOI:10.1007/s11802-014-2078-7 |
Gao F, Xu Q, Yang H S. 2010. Seasonal variations of food sources in Apostichopus japonicus indicated by fatty acid biomarkers analysis. Journal of Fisheries of China, 34(5): 760-767.
(in Chinese with English abstract) DOI:10.3724/SP.J.1231.2010.06768 |
Gao S, Pan L Q, Huang F, Song M S, Tian C C, Zhang M Y. 2019. Metagenomic insights into the structure and function of intestinal microbiota of the farmed pacific white shrimp (litopenaeus vannamei). Aquaculture, 499: 109-118.
DOI:10.1016/j.aquaculture.2018.09.026 |
Gerlach S A. 1978. Food-chain relationships in subtidal silty sand marine sediments and the role of meiofauna in stimulating bacterial productivity. Oecologia, 33(1): 55-69.
DOI:10.1007/BF00376996 |
Hammond L S. 1983. Nutrition of deposit-feeding holothuroids and echinoids (Echinodermata) from a shallow reef lagoon, Discovery Bay, Jamaica. Marine Ecology-Progress Series, 10: 297-305.
DOI:10.3354/meps010297 |
Han H, Yi Y H, Li L, Wang X H, Liu B S, Sun P, Pan M X. 2007. A new triterpene glycoside from sea cucumber Holothuria leucospilota. Chinese Chemical Letters, 18(2): 161-164.
DOI:10.1016/j.cclet.2006.12.027 |
Hatmanti A, Purwati P. 2011. Bacteria associated holothurians: the key of habitat preference, diet, and functions. Jurnal Ilmu Dan Teknologi Kelautan Tropis, 3(1): 73-81.
|
Hauksson E. 1979. Feeding biology of Stichopus tremulus, a deposit-feeding holothurian. Sarsia, 64(3): 155-160.
DOI:10.1080/00364827.1979.10411376 |
Hess M, Sczyrba A, Egan R, Kim T W, Chokhawala H, Schroth G, Luo S J, Clark D S, Chen F, Zhang T, Mackie R I, Pennacchio L A, Tringe S G, Visel A, Woyke T, Wang Z, Rubin E M. 2011. Metagenomic discovery of biomassdegrading genes and genomes from cow rumen. Science, 331(6016): 463-467.
DOI:10.1126/science.1200387 |
Huang W, Huo D, Yu Z, Ren C, Jiang X, Luo P, Chen T, Hu C. 2018. Spawning, larval development and juvenile growth of the tropical sea cucumber Holothuria leucospilota. Aquaculture, 488: 22-29.
DOI:10.1016/j.aquaculture.2018.01.013 |
Ibrahim H A H. 2012. Antibacterial carotenoids of three Holothuria species in Hurghada, Egypt. The Egyptian Journal of Aquatic Research, 38(3): 185-194.
DOI:10.1016/j.ejar.2013.01.004 |
Jia X, Wang L, Zhao Y H, Zhang C Y, Li X D. 2020. Soil microbial communities in the rhizosphere of Robinia pseudoacacia L. after being exposed to elevated atmospheric CO2 and cadmium for 4 years. Applied Soil Ecology, 154: 103661.
DOI:10.1016/j.apsoil.2020.103661 |
Kim J, Kim D Y, Yang K H, Kim S, Lee S S. 2019. Ruegeria lutea sp. nov., isolated from marine sediment, Masan Bay, South Korea. International Journal of Systematic and Evolutionary Microbiology, 69(9): 2 854-2 861.
DOI:10.1099/ijsem.0.003568 |
Kitisin T, Suphamungmee W, Meemon K. 2019. Saponin-rich extracts from Holothuria leucospilota mediate lifespan extension and stress resistance in Caenorhabditis elegans via daf-16. Journal of Food Biochemistry, 43(12): e13075.
|
Liu N N, Zhang S S, Zhang W W, Li C H. 2017. Vibrio sp. 33 a potential bacterial antagonist of Vibrio splendidus pathogenic to sea cucumber (Apostichopus japonicus). Aquaculture, 470: 68-73.
DOI:10.1016/j.aquaculture.2016.12.028 |
MacTavish T, Stenton-Dozey J, Vopel K, Savage C. 2012. Deposit-feeding sea cucumbers enhance mineralization and nutrient cycling in organically-enriched coastal sediments. PLoS One, 7(11): e50031.
DOI:10.1371/journal.pone.0050031 |
Mangion P, Taddei D, Conand C, Frouin P. 2004. Feeding rate and impact of sediment reworking by two deposit feeders Holothuria leucospilota and Holothuria atra on a fringing reef (Reunion Island, Indian Ocean). In: Echinoderms: Munchen: Proceedings of the 11th International Echinoderm Conference. London: CRC Press.
|
Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal, 17(1): 10-12.
DOI:10.14806/ej.17.1.200 |
McLellan S L, Huse S M, Mueller-Spitz S R, Andreishcheva E N, Sogin M L. 2010. Diversity and population structure of sewage-derived microorganisms in wastewater treatment plant influent. Environmental Microbiology, 12(2): 378-392.
DOI:10.1111/j.1462-2920.2009.02075.x |
Mfilinge P L, Tsuchiya M. 2016. Changes in sediment fatty acid composition during passage through the gut of deposit feeding holothurians: Holothuria atra (Jaeger, 1883) and Holothuria leucospilota (Brandt, 1835). Journal of Lipids, 2016: 4579794.
|
Michio K, Kengo K, Yasunori K, Hitoshi M, Takayuki Y, Hideaki Y, Hiroshi S. 2003. Effects of deposit feeder Stichopus japonicus on algal bloom and organic matter contents of bottom sediments of the enclosed sea. Marine Pollution Bulletin, 47(1-6): 118-125.
DOI:10.1016/S0025-326X(02)00411-3 |
Moriarty D J W. 1982. Feeding of Holothuria atra and Stichopus chloronotus on bacteria, organic carbon and organic nitrogen in sediments of the Great Barrier Reef. Australian Journal of Marine and Freshwater Research, 33(2): 255-263.
DOI:10.1071/MF9820255 |
Mußmann M, Pjevac P, Krüger K, Dyksma S. 2017. Genomic repertoire of the Woeseiaceae/JTB255, cosmopolitan and abundant core members of microbial communities in marine sediments. The ISME Journal, 11(5): 1 276-1 281.
DOI:10.1038/ismej.2016.185 |
Paliaga P, Korlević M, Ivančić I, Najdek M. 2017. Limited influence of primary treated sewage waters on bacterial abundance, production and community composition in coastal seawaters. Marine Environmental Research, 131: 215-226.
DOI:10.1016/j.marenvres.2017.09.012 |
Paltzat D L, Pearce C M, Barnes P A, McKinley R S. 2008. Growth and production of California sea cucumbers (Parastichopus californicus Stimpson) co-cultured with suspended Pacific oysters (Crassostrea gigas Thunberg). Aquaculture, 275(1-4): 124-137.
DOI:10.1016/j.aquaculture.2007.12.014 |
Pandiyan P, Balaraman D, Thirunavukkarasu R, George E G J, Subaramaniyan K, Manikkam S, Sadayappan B. 2013. Probiotics in aquaculture. Drug Invention Today, 5(1): 55-59.
DOI:10.1016/j.dit.2013.03.003 |
Phillips N W. 1984. Role of different microbes and substrates as potential suppliers of specific, essential nutrients to marine detritivores. Bulletin of Marine Science, 35(3): 283-298.
|
Purcell S W, Samyn Y, Conand C. 2012. Commercially important Sea Cucumbers of the World. FAO Species Catalogue for Fishery Purposes. No. 6. FAO, Rome.
|
Purcell S W. 2010. Managing Sea Cucumber Fisheries with An Ecosystem Approach. FAO Fisheries and Aquaculture Technical Paper No. 520, FAO, Rome.
|
Purcell S, Conand C, Uthicke S, Byrne M. 2016. Ecological roles of exploited sea cucumbers. Oceanography and Marine Biology, 54: 367-386.
|
Rahiman K M M, Jesmi Y, Thomas A P, Hatha A A M. 2010. Probiotic effect of Bacillus NL110 and Vibrio NE17 on the survival, growth performance and immune response of Macrobrachium rosenbergii (de Man). Aquaculture Research, 41(9): e120-e134.
DOI:10.1111/j.1365-2109.2009.02473.x |
Roberts D. 1979. Deposit-feeding mechanisms and resource partitioning in tropical holothurians. Journal of Experimental Marine Biology and Ecology, 37(1): 43-56.
DOI:10.1016/0022-0981(79)90025-X |
Schneider K, Silverman J, Kravitz B, Rivlin T, Schneider-Mor A, Barbosa S, Byrne M, Caldeira K. 2013. Inorganic carbon turnover caused by digestion of carbonate sands and metabolic activity of holothurians. Estuarine, Coastal and Shelf Science, 133: 217-223.
DOI:10.1016/j.ecss.2013.08.029 |
Silva-Aciares F R, Carvajal P O, Mejías C A, Riquelme C E. 2011. Use of macroalgae supplemented with probiotics in the Haliotis rufescens (Swainson, 1822) culture in Northern Chile. Aquaculture Research, 42(7): 953-961.
DOI:10.1111/j.1365-2109.2010.02678.x |
Sloan N A, von Bodungen B. 1980. Distribution and feeding of the sea cucumber Isostichopus badionotus in relation to shelter and sediment criteria of the Bermuda Platform. Marine Ecology-Progress Series, 2: 257-264.
DOI:10.3354/meps002257 |
Smith T B. 1983. Tentacular ultrastructure and feeding behaviour of Neopentadactyla mixta (Holothuroidea: dendrochirota). Journal of the Marine Biological Association of the United Kingdom, 63(2): 301-311.
DOI:10.1017/S0025315400070697 |
Sun Y, Chen D. 1989. The microbial composition of Stichopus japonicus and its physiological property. Oceanologia et Limnologia Sinica, 20(4): 300-307.
(in Chinese with English abstract) |
Uchino Y, Hirata A, Yokota A, Sugiyama J. 1998. Reclassification of marine agrobacterium species: proposals of Stappia stellulata gen. nov., comb. nov., Stappia aggregata sp. nov., nom. rev., Ruegeria atlantica gen. nov., comb. nov., Ruegeria gelatinovora comb. nov., Ruegeria algicola comb. nov., and Ahrensia kieliense gen. nov., sp. nov., nom. rev. The Journal of General and Applied Microbiology, 44(3): 201-210.
DOI:10.2323/jgam.44.201 |
Uthicke S. 1999. Sediment bioturbation and impact of feeding activity of Holothuria (Halodeima) atra and Stichopus chloronotus, two sediment feeding holothurians, at Lizard Island, Great Barrier Reef. Bulletin of Marine Science, 64(1): 129-141.
|
Vidal-Ramirez F, Dove S. 2016. Diurnal effects of Holothuria atra on seawater carbonate chemistry in a sedimentary environment. Journal of Experimental Marine Biology and Ecology, 474: 156-163.
DOI:10.1016/j.jembe.2015.10.007 |
Viyakarn V, Chavanich S, Heery E, Raksasab C. 2020. Distribution of sea cucumbers, Holothuria atra, on reefs in the upper Gulf of Thailand and the effect of their population densities on sediment microalgal productivity. Estuarine, Coastal and ShelfScience, 235: 106514.
DOI:10.1016/j.ecss.2019.106514 |
Wang L, Zhao X W, Xu H C, Bao X Y, Liu X J, Chang Y Q, Ding J. 2018. Characterization of the bacterial community in different parts of the gut of sea cucumber (Apostichopus japonicus) and its variation during gut regeneration. Aquaculture Research, 49(5): 1 987-1 996.
DOI:10.1111/are.13654 |
Wang Y, Liu Y T, Wang J N, Luo T W, Zhang R, Sun J, Zheng Q, Jiao N Z. 2019. Seasonal dynamics of bacterial communities in the surface seawater around subtropical Xiamen island, china, as determined by 16S rRNA gene profiling. Marine Pollution Bulletin, 142: 135-144.
DOI:10.1016/j.marpolbul.2019.03.035 |
Ward-Rainey N, Rainey F A, Stackebrandt E. 1996. A study of the bacterial flora associated with Holothuria atra. Journal of Experimental Marine Biology and Ecology, 203(1): 11-26.
DOI:10.1016/0022-0981(96)02566-X |
Warnecke F, Luginbühl P, Ivanova N, Ghassemian M, Richardson T H, Stege J T, Cayouette M, McHardy A C, Djordjevic G, Aboushadi N, Sorek R, Tringe S G, Podar M, Martin H G, Kunin V, Dalevi D, Madejska J, Kirton E, Platt D, Szeto E, Salamov A, Barry K, Mikhailova N, Kyrpides N C, Matson E G, Ottesen E A, Zhang X N, Hernández M, Murillo C, Acosta L G, Rigoutsos I, Tamayo G, Green B D, Chang C, Rubin E M, Mathur E J, Robertson D E, Hugenholtz P, Leadbetter J R. 2007. Metagenomic and functional analysis of hindgut microbiota of a woodfeeding higher termite. Nature, 450(7169): 560-565.
DOI:10.1038/nature06269 |
Wen B, Gao Q F, Zhang C, Dong S L, Yu H B, Li W D, Li Z M. 2016. Effects of seasonal changes in the composition of pond sediment on food sources of cultured sea cucumber Apostichopus japonicus indicated by fatty acid biomarkers. Journal of Fisheries of China, 40(11): 1 724-1 731.
(in Chinese with English abstract) |
Wen J, Hu C Q, Zhang L P, Fan S G. 2011. Genetic identification of global commercial sea cucumber species on the basis of mitochondrial DNA sequences. Food Control, 22(1): 72-77.
DOI:10.1016/j.foodcont.2010.06.010 |
Xue Y L, Gao F, Xu Q, Huang D J, Wang A M, Sun T. 2019. Study on feeding selection of environmental sediments and digestive function adaptability of Holothuria atra. Oceanologia et Limnologia Sinica, 50(5): 1 070-1 079.
(in Chinese with English abstract) |
Yang H S, Hamel J F, Mercier A. 2015. The Sea Cucumber Apostichopus japonicus: History, Biology and Aquaculture. Academic Press, Cambridge.
|
Yingst J Y. 1976. The utilization of organic matter in shallow marine sediments by an epibenthic deposit-feeding holothurian. Journal of Experimental Marine Biologyand Ecology, 23(1): 55-69.
DOI:10.1016/0022-0981(76)90085-X |
Zhang X C, Nakahara T, Miyazaki M, Nogi Y, Taniyama S, Arakawa O, Inoue T, Kudo T. 2012. Diversity and function of aerobic culturable bacteria in the intestine of the sea cucumber Holothuria leucospilota. The Journal of General and Applied Microbiology, 58(6): 447-456.
DOI:10.2323/jgam.58.447 |
Zhao F Q, Liu Q B, Cao J, Xu Y S, Pei Z S, Fan H F, Yuan Y Q, Shen X R, Li C. 2020. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food and Chemical Toxicology, 135: 110886.
DOI:10.1016/j.fct.2019.110886 |
Zhao P. 2010. Basic Study on Feeding Selectivity of Sea Cucumber Apostichopus japonicus. Chinese Academy of Science, Beijing. (in Chinese with English abstract)
|
 2022, Vol. 40
2022, Vol. 40


