Institute of Oceanology, Chinese Academy of Sciences
Article Information
- NAN Xueliang, WEI Hao, ZHANG Haiyan, NIE Hongtao
- Spatial difference in net growth rate of Yesso scallop Patinopecten yessoensis revealed by an aquaculture ecosystem model
- Journal of Oceanology and Limnology, 40(1): 373-387
- http://dx.doi.org/10.1007/s00343-021-0423-4
Article History
- Received Oct. 30, 2020
- accepted in principle Jan. 5, 2021
- accepted for publication Feb. 25, 2021
China has recorded higher annual aquaculture production than the rest of the world combined since 1991 (FAO, 2020). In 2018, mariculture production in China exceeded 20.31 million tons, accounting for 66% of the global mariculture production (Fishery Administration Bureau of Ministry of Agriculture and Rural Areas et al., 2019; FAO, 2020). For the sustainable development of aquaculture, the strategic choice of China is to accelerate the green development of aquaculture in the new era (Chen, 2019). It is our duty to promote the green development of aquaculture and to accelerate actively the transformation of developing mode in aquaculture (Chen, 2019). Scientific planning and management of culture areas are one of the significant methods to realize green development, which is based on the research on the spatial difference in the net growth rate of cultured organisms.
Yesso scallop is one of the widely cultured economic shellfish species in China (Zhao et al., 2019). Changhai sea area is now the largest Yesso scallop culture area in the North Yellow Sea, including bottom and raft culture (Yuan et al., 2010; Li et al., 2012). In addition to natural predators, the growth of cultured scallops can be affected by overcapacity, rapid temperature changes, and food shortages (Rico-Villa et al., 2009; Jiang et al., 2018; Zhao et al., 2019), resulting in an uneven spatial distribution of harvestable biomass in the areas (Liu et al., 2014; Zhao et al., 2019). The Changhai sea area was affected by the northern Yellow Sea cold water mass, the Yellow Sea warm current, tidal fronts, etc. (Guan, 1963; Chen, 2009; Nan et al., 2020), and it encompasses a rich array of physical and biological processes. The impact of the spatial difference of environmental dynamics on the bottom cultured Yesso scallop needs scientific evaluation. Several studies conducted field surveys to research the Yesso scallop growth in some sites in the study area (Jiang, 2013; Zhang et al., 2017; Jiang et al., 2020). However, the research on the net growth rate in most areas is not enough, which affects the scientific planning and management of the aquaculture area. Therefore, it is necessary to simulate and analyze the key factors influencing scallop growth in different areas and different periods after one year of uniform seeding of scallop culture and find out the reason for the influence on spatial differences in the net growth rate.
The individual growth model based on the dynamic energy budget (DEB) theory (Kooijman, 2010), named the DEB model, is a widely used model of methodology for quantifying individual shellfish growth. The theory has been applied to a variety of shellfish species based on observed temperature and food availability (Pouvreau et al., 2006; van der Veer et al., 2006; van der Meer and Kooijman, 2014; Lavaud et al., 2017; Stavrakidis-Zachou et al., 2019; Jiang et al., 2020). However, due to the sporadic observation in space or time under most circumstances, it is hard to copy the temperature and food availability of the culture. Thus, the uncertainty may be large in the modeled results. The latest development in this field involves coupling the DEB model with a 2D physical-biogeochemical model (Guyondet et al., 2010; Filgueira et al., 2014) or a 3D hydrodynamic model and box model of food at the bottom boundary layer (Zhao et al., 2019). However, water temperature and food availability in the North Yellow Sea is largely controlled by the hydrodynamic and thermal conditions, including the circulation, solar radiation, seasonal stratification, freshwater discharge, among others. Using the bottom box model alone cannot replace the dynamic changes of food availability in the natural condition. Therefore, our research team built a physical-biological coupling model. The model is based on a 3D high-resolution, regional circulation model configured with the Regional Ocean Modeling System (ROMS) (Shchepetkin and McWilliams, 2005) and an ecological model called the CoSiNE (carbon, silicate, and nitrogen ecosystem) model (Xiu and Chai, 2014). All the components of ROMSCoSiNE are affected by physical processes, such as advection and diffusion (Zhou et al., 2017). ROMSCoSiNE model provided more detailed 3D data of temperature and food availability, which serves as the forcing conditions to the DEB model to enhance our estimation on the net growth rate of Yesso scallop.
The objective of this study is to develop a Yesso scallop culture ecosystem (YeSCE) model considering physical-biological condition and individual growth of Yesso scallop to guide the aquaculture in the Changhai sea area and possibly the other places in the future, to predict the areas with little-to-no data, and determine the influence factors of spatial difference in net growth rate of scallops.
2 MATERIAL AND METHOD 2.1 Study area and field dataChanghai sea area is located in the North Yellow Sea to the east of Liaodong Peninsula (Fig. 1a, red box). The area is affected by the Yellow Sea Warm Current in winter and the North Yellow Sea Cold Water Mass in summer (Guan, 1963; Chen, 2009). The main study area for YeSCE model includes the aquaculture area of Zhangzi Island, Changshan Islands, and Haiyang Island (Fig. 1b). Traditional raft culture (blue polygon) and bottom culture areas (yellow polygon) are shown in Fig. 1b (Yuan et al., 2010; Li et al., 2012). In this study, it is assumed that the whole sea area was bottom-cultured, and the results can be used as a basis for future planning.

|
| Fig.1 Map of the study area, observation stations, and representative sites a. topography and CTD stations (black dots) and the Changhai sea area (red frame); b. scallop observation stations, acoustic Doppler current profiler (ADCP) location, and representative sites. |
The colormap represents the water depth in Fig. 1a, and the black dots represent the survey stations of the National Nature Science Foundation of China (NSFC) Ship-time Sharing Project in the North Yellow Sea. The conductivity-temperature-depth data measured with a fluorometer (CTD; RBR 620) from September 10 to 12, 2017, the vertical temperature profiles at every transect station with a sampling rate of 6 Hz. The temperature and chlorophyll a from the bottom layer were measured by SBE 911 on July 9–11, 2016. Station ZZD (red triangle in Fig. 1b) includes a bottom-mooring frame that carries an upward-facing acoustic Doppler current profiler (ADCP, RDI 300K) and a CTD (RBR 420), which collects temperature and current data near the bottom layer from July 10 to August 26, 2017, the sampling frequency of 15 s (Nan et al., 2020). The temperature and chlorophyll-a data were daily averaged for the following analysis. The survey stations for scallop growth are shown in red stars in Fig. 1b. Shell length and flesh dry weight were recorded in station ZZD_01 from December 2012 to November 2014 (Jiang et al., 2020), with which the half-saturation coefficient was calibrated; and temperature and chlorophyll a were recorded daily (Jiang et al., 2020). Shell lengths were recorded in stations ZZD_02 and ZZD_03 from December 2012 to August 2013 (Jiang, 2013), with which the scallop growth was determined. Temperature and chlorophyll a were monthly measured at 2-m depth in station ZZD_04 from January to December 2007 (Yuan et al., 2010). In addition, three representative sites (red squares in Fig. 1b) S1 (16 nautical miles south of Zhangzi Island), S2 (near Zhangzi Island), and S3 (16 nautical miles north of Zhangzi Island and the location the same to station ZZD_04) (Fig. 1b) selected in latitudinal direction were used to analyze the spatial and temporal differences of the relationship among temperature, food supply, and scallop growth in section Discussion later.
2.2 Overview of the YeSCE modelThe Yesso scallop culture ecosystem (YeSCE) model consists of a hydrodynamic model (ROMS), an ecosystem model (CoSiNE), and an offline DEB model for Yesso scallop. ROMS and CoSiNE were run simultaneously at 240-s time interval. The realm of ROMS-CoSiNE covered the Bohai Sea (BHS), the Yellow Sea, and part of the East China Sea (Fig. 2), in homogeneous 1/24° resolution in horizontal direction and 30 terrain-based vertical levels. Model temperature, salinity, nutrients (nitrate, phosphate, and silicate), and dissolved oxygen were initialized using data from the World Ocean Atlas 2013 V2 (WOA13 V2; Boyer et al., 2015). The initial condition for chlorophyll a was prescribed from the hydrodynamical and biogeochemical model NEMOPISCES (Person et al., 2019); and the model was forced with the climatological air-sea fluxes of momentum, wind stress, heat flux, and freshwater flux derived from the ERA-Interim reanalysis (Dee et al., 2011). An Orlanski-type radiation condition was applied at the open boundaries for temperature, salinity, and baroclinic velocities (Zhou et al., 2017). The temperature, salinity, water level, and velocity fields were prescribed using the Hybrid Coordinate Ocean Model (HYCOM; Cummings, 2005) simulation outputs; and the model was forced (data input) at outer boundary by M2, S2, K2, N2, K1, O1, Q1, and P1 tide (Luo et al., 2019). Major rivers that surround the study realm in the model included the Changjiang (Yangtze) River, Huanghe (Yellow) River, Liaohe River, Luanhe River, Yalu River, Qiantang River, and Huaihe River in China, and Han River in Korea Peninsula. Monthly-mean runoff of the Changjiang River was used from the measurements at Datong hydrologic station and the monthly-mean nutrient from Global-News model (Wang et al., 2015), while monthly-mean values of the other rivers are taken from previous studies (Zhang, 1996; Liu et al., 2009; Tong et al., 2015). The model consists of 11 state variables, including four on nutrients (phosphate, silicate, nitrate, and ammonium), two on phytoplankton groups (picophytoplankton and diatoms), two on grazers (microzooplankton and mesozooplankton), two on class of detritus (small/suspended and large/sinking), and oxygen. All the state variables are affected by physical processes, such as advection and diffusion (Zhou et al., 2017). Phytoplankton, zooplankton, and organic detritus are the main food sources for filter scallops (Aya and Kudo, 2010), in the unit of (mmol N)/m3. Using the unit conversion in terms of carbon to nitrogen (C/N) ratio at 6.625 (Redfield et al., 1963), the food availability in this study was represented as particulate organic carbon ((mg C)/m3). The simulated phytoplankton concentration ((mmol N)/m3) was converted to chlorophyll-a concentration (mg/m3) using the ratio of 1.58-g chlorophyll a per mole nitrogen (Cloern et al., 1995; Zhou et al., 2017). The chlorophyll a was often used to present the biomass of phytoplankton or the food filtered and digested by bivalve mollusks (Pouvreau et al., 2006). The rationality of food availability was explained indirectly by verifying chlorophyll-a concentration. The daily-averaged temperature and food availability of the bottom layer from December 1, 2012 to November 30, 2013 were recorded and used to run the offline DEB model.

|
| Fig.2 Conceptualization of the YeSCE model The state variables are nutrients (phosphate, silicate, nitrate, and ammonium), picophytoplankton (S1), diatom (S2), microzooplankton (Z1), mesozooplankton (Z2), chlorophyll a corresponding to micro phytoplankton (Chl1), chlorophyll a corresponding to diatom (Chl2), detritus (small/suspended and large/sinking), Reserves (E), structure (V), and maturity & reproduction (ER). |
Equations of DEB model were solved using the fourth-order Runge-Kutta numerical integration scheme (Kooijman, 2010) with a time step of 1 day. The DEB model simulates the feeding, digestion, maintenance, maturation, growth, reproduction, and aging processes (Stavrakidis-Zachou et al., 2019), the maintenance energy is defined as the (mean) energy requirement of an organism, excluding the production processes of growth, reproduction, and development (Kooijman, 2010). These processes are predominantly influenced by water temperature and food availability (Kooijman, 2010). The state variables, energy fluxes, and dynamics of the standard DEB model are summarized in Table 1. Two different Arrhenius relationships (K1 and K2) were used for scallop ingestion and respiration rates (Bourlès et al., 2009). Most of the key parameters applied in the DEB model were identified by previous experiments in Zhangzi Island conducted by Zhang et al.(2016, 2017). Only the half-saturation coefficient of food was calibrated. For a more comprehensive description of the DEB theory and the full list of the equations and the nomenclature, please refer to Kooijman (2010).
The food uptake of scallops in the DEB model follows Holling's type II functional response: f=X/(X+XK), where X is the food availability and XK is the half-saturation coefficient, which is the only parameter that requires calibration. Observations of the shell lengths and dry flesh weights of scallops in station ZZD_01 (Fig. 1b; Jiang et al., 2020) were used as the standard. Scallop seeds, with a mean (±SD) shell length of 3.80±0.52 cm and dry flesh weight of 0.23±0.08 g, were spread abroad the bottom layer on December 1, 2012 (Jiang et al., 2020). The model was calibrated against the field observation by minimizing the weighted sum of squared residuals (SSR) using the formulation from Troost et al. (2010). The food availability that corresponds to the minimum SSR was selected as the XK for YeSCE modeling.
Based on the optimal XK, the observed values of shell length (R=0.97) and dry flesh weight (R=0.94) exhibited a significant correlation with the modeled values. The model can well simulate the growth process of the shell length (Fig. 3a) and the change in the energy state of the scallop (Fig. 3b).

|
| Fig.3 The observation (blue dots with bars for standard deviation) from December 1, 2012 to November 30, 2014, and the simulation (black line) a. shell length; b. dry flesh weight. |
Based on the spatial resolution of the YeSCE model, 810 grid points in the bottom layer in the study area were set up. Yesso scallop seed in each grid point were corresponded with the observations from Jiang et al. (2020), being 3.80 cm in shell length and 0.23 g in dry flesh weight. The model was run for one year from 1 December 2012 to 30 November 2013 to be consistent with scallop growth data (Jiang, 2013). The model could output the time series of temperature, food availability, shell length, and biomass at each grid point near bottom layer. Biomass is the dry flesh weight of Yesso scallop in a unit area. The net growth rate refers to the changes in biomass over one year. In the study, we explained the net growth rate as the biomass difference from December 1, 2012 to November 30, 2013.
2.5 Suitable growth daysThe match-mismatch condition between temperature and food availability affects the growth of organisms (Laurel et al., 2011; Scharf et al., 2015). We defined suitable growth days (SGD) as the number of days of suitable temperature and sufficient food, which means that the temperature and the food availability were highly matched. The suitable temperature refers to the value in the temperature tolerance range for ingestion (0–20 ℃) (Zhang et al., 2016, 2017). Sufficient food refers to the food availability (X) no less than the halfsaturation coefficient (XK).
3 RESULT 3.1 Evaluation of the YeSCE model 3.1.1 Bottom temperatureTemperature data shown in Fig. 4a were collected with a calibrated CTD (RBR 620) from September 10 to 12, 2017. The distribution of the simulated bottom temperature (background) and observations (points) was consistent, revealing a significant correlation between observations and simulations. The model could better reproduce the bottom temperature nearshore than the offshore, and could characterize the cold-water mass and tidal front in the North Yellow Sea (Fig. 4a). A more thorough assessment of simulation-observation agreement was conducted quantitatively using the Taylor diagram (Taylor, 2001). Temperature data in the bottom layer were from two large-scale surveys in July 9–11, 2016 and September 10–12, 2017, bottom-mooring in station ZZD from July 10 to August 26, 2017, and ZZD_01 from December 2012 to November 2014. The temperature was recorded at 2-m depth in station ZZD_04 from January to December 2007. In addition to the correlation and root mean squared difference (RMSD), standard deviations of simulations and observations were calculated in the diagram for each survey (Fig. 4b). Note that the standard deviation was normalized by that of observation data to fit all comparisons into one diagram. The correlation between the bottom temperature observation value and the simulation value was significant (R > 0.80). Therefore, the model simulated the bottom temperature accurately (Fig. 4b).

|
| Fig.4 Bottom temperature of simulation (colormap) and observations (dots) on September 10-12, 2017 (a), and Taylor diagram comparing bottom temperature estimates from the observation (blue dot) and the simulations (red dots) (b) In (a), the colormap is the average results of simulations from September 10 to 12, 2017, and the domain of Changhai sea area (red frame) and survey stations are also shown. In (b), the radial distance from the origin is proportional to the ratio of model and observation standard deviations (black curves), and the azimuthal position corresponds to the correlation between model and observation fields (blue dashed lines). The distance from each model value to the observation value is proportional to the normalized RMSD between observation and simulated fields (purple curves). |
The distribution of chlorophyll a from YeSCE model matched the CTD (RBR 620) observations from September 10 to 12, 2017. Generally, the simulated chlorophyll a in the bottom layer gradually decreased with an increase in the offshore distance (Fig. 5a). A more thorough assessment of simulation-observation agreement was conducted quantitatively using the Taylor diagram (Taylor, 2001). Chlorophyll-a data in the bottom layer were from two large-scale surveys on July 9–11, 2016 and September 10–12, 2017, and ZZD_01 from December 2012 to November 2014. Chlorophyll a was recorded at 2-m depth in ZZD_04 from January to December 2007. In the diagram, in addition to the correlation and RMSD, standard deviations of simulations and observations were calculated for each survey (Fig. 5b). The statistics showed that the model was capable of modeling chlorophyll a in the study area (Fig. 5b). The concentration of chlorophyll a has often been used to present the biomass of phytoplankton or food filtered and digested by bivalve mollusks (Pouvreau et al., 2006). Therefore, an accurate simulation of concentration of chlorophyll a is essential to improve the data quality for growth modeling.

|
| Fig.5 The bottom concentration of chlorophyll a simulated (colormap) and observed (dots) in September 2017 (a), and the Taylor diagram comparing bottom concentration of chlorophyll a estimates from the observation (blue dot) and the simulations (red dots) (b) In (a), the colormap is the average of simulations from September 10 to 12, 2017, and the domain of Changhai sea area (red frame) and survey stations are also shown. In (b), the radial distance from the origin is proportional to the ratio of model and observation standard deviations (black curves), and the azimuthal position corresponds to the correlation between model and observation fields (blue dashed lines). The distance from each model value to the observation value is proportional to the normalized RMSD between observation and simulated fields (purple curves). |
Based on the observations of Jiang (2013) at stations ZZD_02 and ZZD_03 (Fig. 1b), YeSCE model simulated the shell length from December 1, 2012 to November 30, 2013. The initial value of shell length was 3.80 cm, which was the same as that in the observation. Reasonable agreement between the simulation results and field observation results verified the reliability of the model (Fig. 6). The modeled shell length results were low in March, mainly due to the insufficient food supply from January to March.

|
| Fig.6 Comparison of simulated (line) and observed (dots with bars for standard deviation) shell lengths in stations ZZD_02 (red) and ZZD_03 (black) |
The distribution of simulated monthly average bottom temperature in the study area from December 2012 to November 2013 showed apparent seasonal variations and differences from north to south (Fig. 7). The simulated temperature in the northern part of the study area changed sharply, with an annual temperature difference of more than 28 ℃, whereas the difference in the southern part of the study area was relatively small. The temperature in the northern shallow-water area was higher than that in the south in summer but lower in winter. The Yellow Sea Cold Water Mass (< 10 ℃) affected the southern areas with a depth of more than 30 m in June, then gradually retreats to deep-water areas and disappeared in October. The shallow-water area temperature dropped substantially in winter and remained above 4 ℃ in the deep-water area. In January, the water temperature in the north of the study area dropped to 0 ℃. In February and March, it fell into 4 ℃. The extreme high/low water temperature in summer/winter could be the major factor that affected the scallop growth.

|
| Fig.7 Distribution of monthly averaged bottom temperature from December 2012 to November 2013 The 4 ℃ and 10 ℃ isotherms (black line), scallop observation stations (red stars), ADCP location (red triangle), and representative sites (red squares) are also shown. |
The distribution of simulated monthly average food in the study area from December 2012 to November 2013 exhibits seasonal variation (Fig. 8). Food availability over the entire study area were lower than XK from November to February, during which shellfish were restricted by food availability, and grew slowly or stopped growing. The food availability increased from March, peaked in April–May, remained high in June–July, and then gradually decreased. Furthermore, food availability were significantly higher in the north than in the south. In the southern deep-water area, the food availability was lower than XK, which hampered the growth of scallops. High food availability were observed near islands (such as Zhangzi Island). Here, the scallops grow rapidly from April to May (Fig. 8e & f) because the food availability was approximately twice that of the surrounding area.
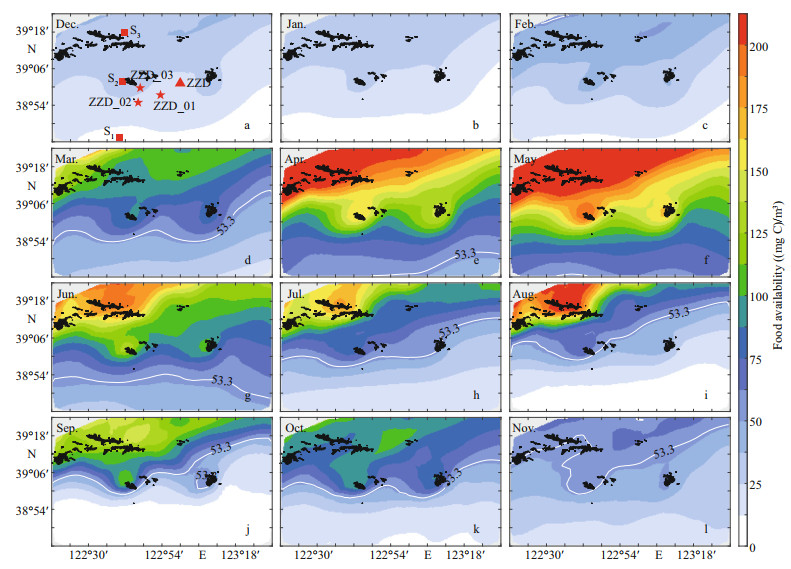
|
| Fig.8 Distribution of monthly averaged food availability from December 2012 to November 2013 The half-saturation coefficient of food availability (white line), scallop observation stations (red stars), ADCP location (red triangle), and representative sites (red squares) are also shown. |
The YeSCE model was applied to the Changhai sea area in the North Yellow Sea to determine the influence of natural factors other than the initial seeding density from December 1, 2012 to November 30, 2013. The initial shell length and dry flesh weight were 3.80 cm and 0.23 g, respectively. The distribution of shell length was high in the north and low in the south, with high values near the islands. At the end of the simulation near Zhangzi Island, the shell length was more than double of the initial shell length up to 8.00 cm (Fig. 9a). Similarly, the net growth rate was also the highest near Zhangzi Island (Fig. 9b). Therefore, the factors on the spatial difference of the net growth rate need to be determined.

|
| Fig.9 Distribution of shell length (a) and net growth rate (b) In (a), scallop observation stations (red stars), ADCP location (red triangle), and representative sites (red squares) are also shown. |
The net growth rate refers to the changes in biomass over one year; therefore, the difference in net growth rate was influenced by biomass in different seasons. Based on the spatial distribution of the net growth rate, three representative sites (S1, S2, and S3, Fig. 1b) were selected to analyze the spatial and temporal differences of the relationship among temperature, food availability, and scallop growth. Clear difference was revealed in biomass contribution from the three representative sites. The biomass of 0.78 g/m2 in site S1 at the end of the simulation was the lowest among the three sites (Fig. 10a). The temperature varied between 3 to 15 ℃ and food availability was below 60 (mg C)/m3 (Fig. 10b). At the end of the simulation, the biomass in site S2 was 2.60 g/m2 (Fig. 10d), which was higher than that of S3 (2.30 g/m2) (Fig. 10g). The duration of temperature lower than 0 ℃ or higher than 20 ℃ in site S3 was longer than that in site S2 (Fig. 10e & h). The food availability varied between 25 to 220 (mg C)/m3 in site S2, and that of site S3 varied between 30 to 300 (mg C)/m3 (Fig. 10e & h).

|
| Fig.10 Time series of biomass (a, d, g), temperature and food availability (b, e, h), and factors influencing scallop growth (c, f, i) in three representative sites (S1, S2, and S3) The first row shows the results of S1, the second is for S2, and the third is for S3. f is the dimensionless functional response, which can vary between 0 and 1. K1 and K2 represent the temperature dependence of ingestion and respiration respectively. |
The distribution of net growth rate was high in the north and low in the south, with high values near the islands. The spatial differences of net growth rate were the same as the result of the potential for scallop aquaculture development (Liu et al., 2014). It is important to find out the reasons for spatial differences in the growth rate. f is the dimensionless functional response, which can vary between 0 and 1, and smaller values indicate greater restriction (van der Meer, 2006). The temperature dependence (K1, K2) is a physiological rate at ambient temperature; the smaller the value, the greater the restriction (Bourlès et al., 2009). There was a clear difference in the biomass contribution of the three representative sites (Fig. 10a, d, & g). Food restriction was the key factor influencing the growth of scallops in site S1 (Fig. 10e). In other words, the low net growth rate area south of the study area was caused chiefly by the lower food availability.
The temperature dependence was the key limiting factor affecting the scallop growth in station S2 during January to July 2013, which is similar to the previous study near Zhangzi Island (Zhao et al., 2019). The biomass in site S2 at the end of the simulation was higher than that of site S3. However, the biomass in site S3 (0.40–1.97 g/m2) was higher than that in site S2 (0.45–1.84 g/m2) from April to July (Fig. 10d & j). In this period, the bottom temperature limitation of the two stations was similar, but the food availability in site S3 was almost twice that of site S2 (Fig. 10e & h). This raises the question of why the biomass was lower in site S3. The reason is that the duration of temperature below 0 ℃ in site S3 was longer than that in site S2 from January to March, and the biomass in site S2 was 0.45 g/m2, whereas that in site S3 was 0.40 g/m2 by April. From July to October, the duration of temperature above 20 ℃ in site S3 was longer than that in site S2. In this period, temperature restriction was significant. The changes in f in the two sites were similar. When the food availability was greater than XK, the number of days of a suitable temperature determined the final biomass of scallops.
4.2 Quantifying the effects of food and temperature on scallop growthBased on the relationship between biomass, temperature, and food availability (Fig. 10), the match-mismatch condition between temperature and food availability affected the growth of organisms (Laurel et al., 2011; Scharf et al., 2015). Meanwhile, the suitable growth days (SGD) represent the number of days during which the temperature and the food availability are highly matched (Laurel et al., 2011). The linear regression of SGD and the biomass at the end of the simulation (BIO) correlates well (BIO=1.19×10-2×SGD+0.14, R2=0.96) (Fig. 11a). The equation can be used to express the change in biomass at the end of the simulation. The value 1.19×10-2×SGD represents the contribution of suitable growth days to scallop growth (CSGD). The high-value area of CSGD extends from Zhangzi Island to the northeast of the study area, which is similar to the distribution of the net growth rate. Therefore, the length of SGD is the main factor affecting the spatial difference in the net growth rate of Yesso scallop.

|
| Fig.11 The relationship between regressed and modeled biomass (a) and the contribution of suitable growth days to scallop growth (CSGD) (b) |
The contribution of SGD to scallop growth well displayed the spatial differences of net growth rate from December 1, 2012 to November 30, 2013 (Fig. 11), the interannual variation in the scallop growth will be studied in the future. In this study, we considered only the match condition (Laurel et al., 2011) under which the value in temperature tolerance range for ingestion and the food availability (X) is no less than the half-saturation coefficient (XK). In other words, we considered that the period of scallop growth were not the slow growth or weight-loss period (Scharf et al., 2015). In the future, we will add mismatch conditions by analyzing the relationship among temperature, food availability, and biomass to make the fitting results more reasonable and find out some parameters that are easy to obtain in practice to characterize the spatial differences of net growth rate and forecast its interannual variations. Furthermore, YeSCE model can be used to study the interactions between scallop carrying capacity, seeding density, and rotational culture in different regions by changing the initial seeding density and distribution. YeSCE model still needs to be improved in terms of energy dissipation due to biological stress and death due to the invasion of natural predators. Future work should be based on further biological experiments on scallops.
5 CONCLUSIONThe YeSCE (Yesso scallop culture ecosystem) model could reasonably simulate the environment and scallop growth in the Changhai sea area. Scallop growth varied seasonally, showing two slow-growth periods from January to March and July to September, which was resulted predominantly from bottom water temperature. The remaining months comprised a fast-growth period; however, the growth rate from October to December was lower than that from April to June due to food availability. The annual net growth rate was high in the north, low in the south, and notably high near the islands. Based on the correlation analysis, the spatial differences of the net growth rate were predominantly affected by the duration of suitable growth days.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTThe transect observations were collected onboard of R/V Dongfanghong 2 implementing the open research cruise NORC2017-01 supported by the NSFC Ship-time Sharing Project. We acknowledge the crew members of R/V Liaokeyu 19 from the Zhangzi Island group for their support. We appreciate the help of Simeng QIAN, Chenyi LUO, and Guangyue ZHANG in the preparation of the ecosystem model.
Aya F A, Kudo I. 2010. Isotopic shifts with size, culture habitat, and enrichment between the diet and tissues of the Japanese scallop Mizuhopecten yessoensis (Jay, 1857). Marine Biology, 157(10): 2 157-2 167.
DOI:10.1007/s00227-010-1480-y |
Bourlès Y, Alunno-Bruscia M, Pouvreau S, Tollu G, Leguay L, Arnaud C, Goulletquer P, Kooijman S A L M. 2009. Modelling growth and reproduction of the Pacific oyster Crassostrea gigas: advances in the oyster-DEB model through application to a coastal pond. Journal of Sea Research, 62(2-3): 62-71.
DOI:10.1016/j.seares.2009.03.002 |
Boyer T, Locarnini R A, Zweng M M, Mishonov A V, Reagan J R, Antonov J I, Garcia H E, Baranova O K, Johnson D R, Seidov D, Biddle M M, Hamilton M. 2015. Changes to calculations of the World Ocean Atlas 2013 for version 2. http://data.nodc.noaa.gov/woa/WOA13/DOC/woa13v2_changes.pdf. Accessed on 2020-02-23.
|
Chen C T A. 2009. Chemical and physical fronts in the Bohai, Yellow and East China seas. Journal of Marine Systems, 78(3): 394-410.
DOI:10.1016/j.jmarsys.2008.11.016 |
Chen J Y. 2019. Seize opportunities to accelerate the green development of aquaculture. China Fishery Quality and Standards, 9(4): 1-4.
(in Chinese with English abstract) |
Cloern J E, Grenz C, Vidergar-Lucas L. 1995. An empirical model of the phytoplankton chlorophyll: carbon ratio-the conversion factor between productivity and growth rate. Limnology and Oceanography, 40(7): 1 313-1 321.
DOI:10.4319/lo.1995.40.7.1313 |
Cummings J A. 2005. Operational multivariate ocean data assimilation. Quarterly Journal of the Royal Meteorological Society, 131(613): 3 583-3 604.
DOI:10.1256/qj.05.105 |
Dee D P, Uppala S M, Simmons A J, Berrisford P, Poli P, Kobayashi S, Andrae U, Balmaseda M A, Balsamo G, Bauer B, Bechtold P, Beljaars A C M, van de Berg L, Bidlot J, Bormann N, Delsol C, Dragani R, Fuentes M, Geer A J, Haimberger L, Healy S B, Hersbach H, Hólm E V, Isaksen L, Kållberg P, Köhler M, Matricardi M, McNally A P, Monge-Sanz B M, Morcrette J J, Park B K, Peubey C, de Rosnay P, Tavolato C, Thépaut J N, Vitart F. 2011. The ERA-Interim reanalysis: configuration and performance of the data assimilation system. Quarterly Journal of the Royal Meteorological Society, 137(656): 553-597.
DOI:10.1002/qj.828 |
FAO. 2020. The state of world fisheries and aquaculture 2020. http://www.fao.org/state-of-fisheries-aquaculture/en/.
|
Filgueira R, Guyondet T, Comeau L A, Grant J. 2014. A fully-spatial ecosystem-DEB model of oyster (Crassostrea virginica) carrying capacity in the Richibucto Estuary, Eastern Canada. Journal of Marine Systems, 136: 42-54.
DOI:10.1016/jjmarsys.2014.03.015 |
Fishery Administration Bureau of Ministry of Agriculture and Rural Areas, National Aquatic Technology Promotion Center, China Fisheries Society. 2019. China Fishery Statistical Yearbook of 2019. China Agriculture Press, Beijing, China. p. 1-172. (in Chinese)
|
Guan B X. 1963. A preliminary study of the temperature variations and the characteristics of the circulation of the Cold Water Mass of the Yellow Sea. Oceanologia et Limnologia Sinica, 5(4): 255-284.
(in Chinese) |
Guyondet T, Roy S, Koutitonsky V G, Grant J, Tita G. 2010. Integrating multiple spatial scales in the carrying capacity assessment of a coastal ecosystem for bivalve aquaculture. Journal of Sea Research, 64(3): 341-359.
DOI:10.1016/j.seares.2010.05.003 |
Helm M. 2005. Cultured aquatic species information programme. Patinopecten yessoensis (Jay, 1857). http://www.fao.org/fishery/culturedspecies/Patinopecten_yessoensis/en. Accessed on 2020-02-23.
|
Jiang W W, Lin F, Du M R, Fang J G, Fang J H, Gao Y P, Wang X Q, Li F X, Dong S P, Hou X, Jiang Z J. 2020. Simulation of Yesso scallop, Patinopecten yessoensis, growth with a dynamic energy budget (DEB) model in the mariculture area of Zhangzidao Island. Aquaculture International, 28(1): 59-71.
DOI:10.1007/s10499-019-00447-6 |
Jiang W W, Lin F, Fang J G, Gao Y P, Du M R, Fang J H, Li W H, Jiang Z J. 2018. Transcriptome analysis of the Yesso scallop, Patinopecten yessoensis gills in response to water temperature fluctuations. Fish & Shellfish Immunology, 80: 133-140.
DOI:10.1016/j.fsi.2018.05.038 |
Jiang X. 2013. Study on the Growth, Food Source, Oxygen Consumption and Ammonia Excretion of Scallop Patinopecten Yessoensis Jay. Nanjing Agricultural University, Nanjing, China. (in Chinese)
|
Kooijman S A L M. 2010. Dynamic Energy Budget Theory for Metabolic Organisation. Cambridge: Cambridge University Press: p. 1-514.
|
Laurel B J, Hurst T P, Ciannelli L. 2011. An experimental examination of temperature interactions in the match-mismatch hypothesis for Pacific cod larvae. Canadian Journal of Fisheries and Aquatic Sciences, 68(1): 51-61.
DOI:10.1139/F10-130 |
Lavaud R, La Peyre M K, Casas S M, Bacher C, La Peyre J F. 2017. Integrating the effects of salinity on the physiology of the eastern oyster, Crassostrea virginica, in the northern Gulf of Mexico through a Dynamic Energy Budget model. Ecological Modelling, 363: 221-233.
DOI:10.1016/j.ecolmodel.2017.09.003 |
Li H B, Liang Y B, Yuan X T. 2012. The effect of raft-culture on distribution of Synchococcus in Changhai waters, Liaoning. Acta Oceanologica Sinica, 34(5): 221-225.
(in Chinese with English abstract) |
Liu S M, Hong G H, Zhang J, Ye X W, Jiang X L. 2009. Nutrient budgets for large Chinese estuaries. Biogeosciences, 6(10): 2 245-2 263.
DOI:10.5194/bg-6-2245-2009 |
Liu Y, Saitoh S I, Radiarta I N, Igarashi H, Hirawake T. 2014. Spatiotemporal variations in suitable areas for Japanese scallop aquaculture in the Dalian coastal area from 2003 to 2012. Aquaculture, 422-423: 172-183.
DOI:10.1016/j.aquaculture.2013.11.033 |
Luo C Y, Nie H T, Zhang H Y. 2019. Spatial variability of parameter sensitivity in the ecosystem simulation of the Bohai Sea and Yellow Sea. Haiyang Xuebao, 41(8): 85-96.
(in Chinese with English abstract) |
Nan X L, Wei H, Fan R F, Yang W. 2020. Rapid changes in the near-bottom temperature of the bottom aquaculture area around the Zhangzi Island in summer. Acta Oceanologica Sinica, 39(5): 46-54.
DOI:10.1007/s13131-020-1605-1 |
Person R, Aumont O, Madec G, Vancoppenolle M, Bopp L, Merino N. 2019. Sensitivity of ocean biogeochemistry to the iron supply from the Antarctic Ice Sheet explored with a biogeochemical model. Biogeosciences, 16(18): 3 583-3 603.
DOI:10.5194/bg-16-3583-2019 |
Pouvreau S, Bourles Y, Lefebvre S, Gangnery A, Alunno-Bruscia M. 2006. Application of a dynamic energy budget model to the Pacific oyster, Crassostrea gigas, reared under various environmental conditions. Journal of Sea Research, 56(2): 156-167.
DOI:10.1016/j.seares.2006.03.007 |
Redfield A C, Ketchum B H, Richards F A. 1963. The influence of organisms on the composition of seawater. In: Hill M N ed. The Composition of Seawater: Comparative and Descriptive Oceanography. The Sea: Ideas and Observations on Progress in the Study of the Seas, 2. Interscience Publishers, New York. p. 26-77.
|
Rico-Villa B, Pouvreau S, Robert R. 2009. Influence of food density and temperature on ingestion, growth and settlement of Pacific oyster larvae, Crassostrea gigas. Aquaculture, 287(3-4): 395-401.
DOI:10.1016/j.aquaculture.2008.10.054 |
Scharf I, Braf H, Ifrach N, Rosenstein S, Subach A. 2015. The effects of temperature and diet during development, adulthood, and mating on reproduction in the red flour beetle. PLoS One, 10(9): e0136924.
DOI:10.1371/journal.pone.0136924 |
Shchepetkin A F, McWilliams J C. 2005. The regional oceanic modeling system (ROMS): a split-explicit, free-surface, topography-following-coordinate oceanic model. Ocean Modelling, 9(4): 347-404.
DOI:10.1016/j.ocemod.2004.08.002 |
Stavrakidis-Zachou O, Papandroulakis N, Lika K. 2019. A DEB model for European sea bass (Dicentrarchus labrax): parameterisation and application in aquaculture. Journal of Sea Research, 143: 262-271.
DOI:10.1016/j.seares.2018.05.008 |
Taylor K E. 2001. Summarizing multiple aspects of model performance in a single diagram. Journal of Geophysical Research: Atmospheres, 106(D7): 7 183-7 192.
DOI:10.1029/2000JD900719 |
Tong Y D, Zhao Y, Zhen G C, Chi J, Liu X H, Lu Y R, Wang X J, Yao R H, Chen J Y, Zhang W. 2015. Nutrient loads flowing into coastal waters from the main rivers of China (2006-2012). Scientific Reports, 5(1): 16678.
DOI:10.1038/srep16678 |
Troost TA, Wijsman J W M, Saraiva S, Freitas V. 2010. Modelling shellfish growth with dynamic energy budget models: an application for cockles and mussels in the Oosterschelde (southwest Netherlands). Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1557): 3 567-3 577.
DOI:10.1098/rstb.2010.0074 |
van der Meer J, Kooijman S A L M. 2014. Inference on energetics of deep-sea fish that cannot be aged: the case of the hagfish. Journal of Sea Research, 94: 138-143.
DOI:10.1016/j.seares.2014.07.007 |
van der Meer J. 2006. An introduction to Dynamic Energy Budget (DEB) models with special emphasis on parameter estimation. Journal of Sea Research, 56(2): 85-102.
DOI:10.1016/j.seares.2006.03.001 |
van der Veer H W, Cardoso J F M F, van der Meer J. 2006. The estimation of DEB parameters for various Northeast Atlantic bivalve species. Journal of Sea Research, 56: 107-124.
DOI:10.1016/j.seares.2006.03.005 |
Wang J N, Yan W J, Chen N W, Li X Y, Liu L S. 2015. Modeled long-term changes of DIN: DIP ratio in the Changjiang River in relation to Chl-a and DO concentrations in adjacent estuary. Estuarine, Coastal and Shelf Science, 166: 153-160.
DOI:10.1016/j.ecss.2014.11.028 |
Xiu P, Chai F. 2014. Connections between physical, optical and biogeochemical processes in the Pacific Ocean. Progress in Oceanography, 122: 30-53.
DOI:10.1016/j.pocean.2013.11.008 |
Yuan X T, Zhang M J, Liang Y B, Liu D, Guan D M. 2010. Self-pollutant loading from a suspension aquaculture system of Japanese scallop (Patinopecten yessoensis) in the Changhai sea area, Northern Yellow Sea of China. Aquaculture, 304(1-4): 79-87.
DOI:10.1016/j.aquaculture.2010.03.026 |
Zhang J H, Wu W G, Liu Y, Lin F, Wang W, Niu Y L. 2017. A dynamic energy budget (DEB) growth model for Japanese scallop Patinopecten yessoensis cultured in China. Journal of Fishery Sciences of China, 24(3): 497-506.
(in Chinese with English abstract) DOI:10.3724/SP.J.1118.2017.16227 |
Zhang J H, Wu W G, Xu D, Ren L H, Niu Y L, Zhao X W. 2016. The estimation of Dynamic Energy Budget (DEB) model parameters for scallop Patinopecten yessoensis. Journal of Fisheries of China, 40(5): 703-710.
(in Chinese with English abstract) |
Zhang J. 1996. Nutrient elements in large Chinese estuaries. Continental Shelf Research, 16(8): 1 023-1 045.
DOI:10.1016/0278-4343(95)00055-0 |
Zhao Y X, Zhang J H, Lin F, Ren J S, Sun K, Liu Y, Wu W G, Wang W. 2019. An ecosystem model for estimating shellfish production carrying capacity in bottom culture systems. Ecological Modelling, 393: 1-11.
DOI:10.1016/j.ecolmodel.2018.12.005 |
Zhou F, Chai F, Huang D J, Xue H J, Chen J F, Xiu P, Xuan J L, Li J, Zeng D Y, Ni X B, Wang K. 2017. Investigation of hypoxia off the Changjiang Estuary using a coupled model of ROMS-CoSiNE. Progress in Oceanography, 159: 237-254.
DOI:10.1016/j.pocean.2017.10.008 |
 2022, Vol. 40
2022, Vol. 40



