Institute of Oceanology, Chinese Academy of Sciences
Article Information
- XIAO Yunlu, XIAO Ning, ZENG Xiaoqi
- Benthodytes tetrapapillata sp. nov., a new elasipodid sea cucumber (Elasipodida: Psychropotidae) from a seamount in the Western Pacific Ocean
- Journal of Oceanology and Limnology, 41(5): 1978-1987
- http://dx.doi.org/10.1007/s00343-022-2220-0
Article History
- Received May 16, 2022
- accepted in principle Jun. 30, 2022
- accepted for publication Aug. 18, 2022
2 Department of Marine Organism Taxonomy & Phylogeny, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Laoshan Laboratory, Qingdao 266237, China;
5 Shandong Province Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
6 Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao 266003, China
Holothurians (Echinodermata, Holothuroidea) are one of the most common benthic fauna on the deep-sea seafloor, widely distributed among all oceans, and occur in various marine environments (Hansen, 1975; Billett, 1988; Gebruk, 1990; Tyler et al., 1996; Rogacheva et al., 2009; Gebruk et al., 2010). At present, there are more than 1 700 described species of holothurians in the world (Paulay and Rogacheva, 2022), and echinoderm species are most widely distributed in the Indo-West Pacific Ocean (Yang et al., 2016).
The order Elasipodida and the family Psychropotidae were established according to the reports of the Challenger Expedition (Théel, 1882). Despite a comprehensive and systematic revision of Psychropotidae by Hansen (1975), the family was still least-known by the taxonomists (Rogacheva et al., 2009). Currently, Psychropotidae includes three valid genera: Psychropotes, Benthodytes, and Psycheotrephes, all erected by Théel (1882).
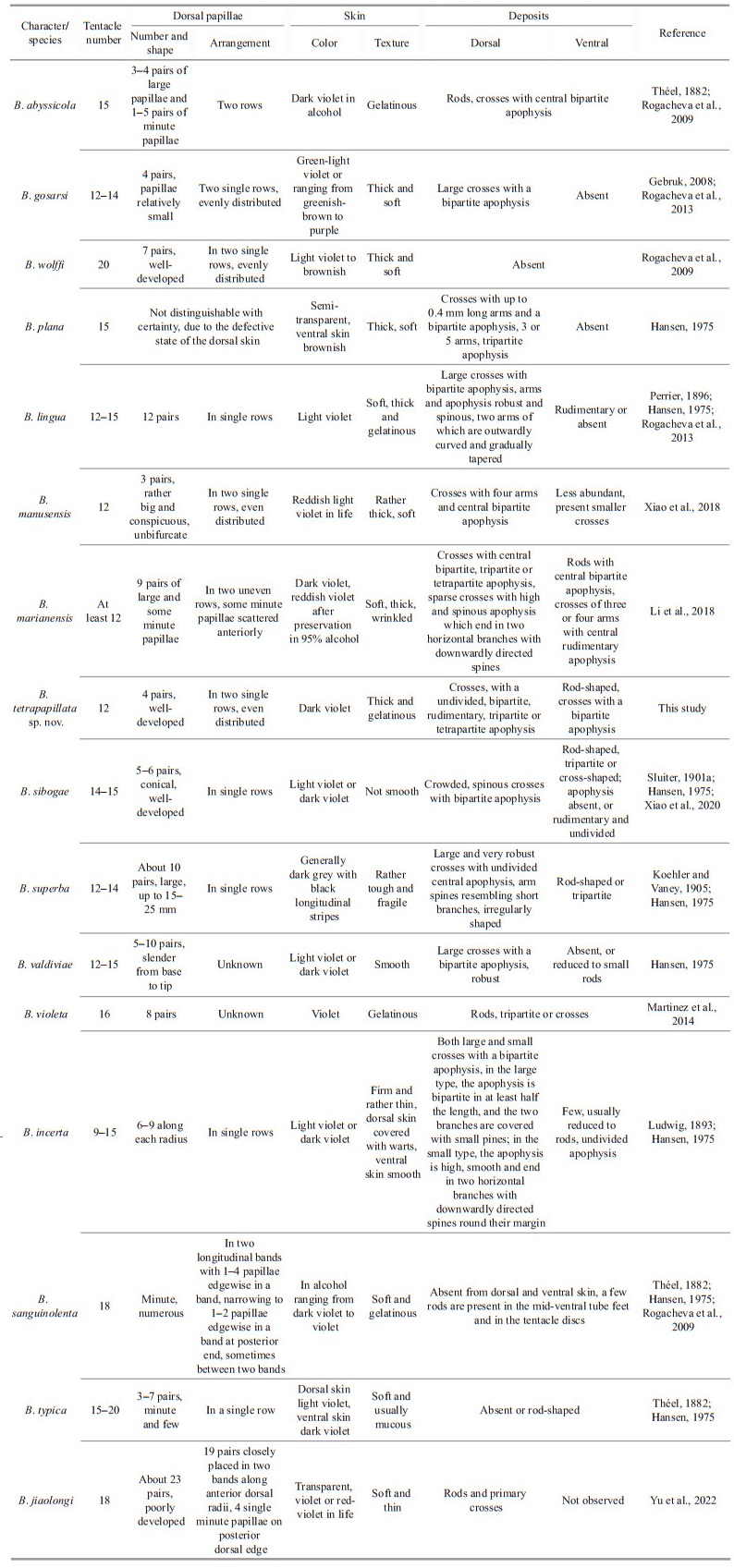
Benthodytes (Echinodermata, Holothuroidea, Elasipodida) differs from the other two genera in a dorsal anus, soft and pliable tentacle discs and circum-oral papillae. Eight species of Benthodytes were described by Théel (1882). Among them, three were accepted after several revisions by taxonomists: Benthodytes abyssicola Théel, 1882, Benthodytes sanguinolenta Théel, 1882, and Benthodytes typica Théel, 1882, of which B. typica was used as the type species to describe this genus. Subsequently, Ludwig (1893), Perrier (1896), Sluiter (1901a), and Koehler and Vaney (1905) described four new species. Hansen (1975) described eight species, including two new species. After that, there were few reports on the taxonomy of Benthodytes. In recent years, more new species have been discovered (Gebruk, 2008; Rogacheva et al., 2009; Martinez et al., 2014; Li et al., 2018; Xiao et al., 2018; Yu et al., 2021, 2022). To date, a total of 15 valid species from Benthodytes have been recorded (WoRMS, 2022), including Benthodytes abyssicola Théel, 1882; B. typica Théel, 1882; B. sanguinolenta Théel, 1882; B. incerta Ludwig, 1894; B. lingua Perrier R., 1896; B. sibogae Sluiter, 1901; B. superba Koehler & Vaney, 1905; B. plana Hansen, 1975; B. valdiviae Hansen, 1975; B. gosarsi Gebruk, 2008; B. wolffi Rogacheva & Cross in Rogacheva et al., 2009; B. violeta Martinez, Solís-Marín & Penchaszadeh, 2014; B manusensis Xiao, Li & Sha, 2018; B. marianensis Li, Xiao, Zhang & Zhang, 2018, and B. jiaolongi Yu, Zhang, Zhang & Wang, 2022. Most of them are distributed around the tropical Western Pacific Ocean, South Indian Ocean and Atlantic Ocean, and are usually found at depths of 600–8 000 m (Théel, 1882; Ludwig, 1894; Perrier, 1896; Sluiter, 1901a, b; Koehler and Vaney, 1905; Hansen, 1975; Gebruk, 2008; Rogacheva et al., 2009; Li et al., 2018; Xiao et al., 2018).
There are about 30 000 seamounts in the world (Yesson et al., 2011). Seamounts are natural obstacles for currents and have great influence on the variable hydrological environment. The environment changes will further affect the biological community, then relatively independent marine ecosystems are formed around them (Dower and Mackas, 1996; Genin, 2004; Clark et al., 2010). Numerous seamounts are scattered in the Western Pacific Ocean, one of the most oligotrophic oceans on Earth. During the cruise of R/V Kexue in June 2019, some holothurian specimens were obtained. Based on morphological and molecular biology methods, a psychropotid species was identified as new to science: Benthodytes tetrapapillata sp. nov. This study and recent studies suggest a high probability that species of Benthodytes and its allies are around the Western Pacific Ocean deep-sea seamounts (Li et al., 2018; Xiao et al., 2018, 2020; Yu et al., 2021, 2022).
2 MATERIAL AND METHOD 2.1 Sampling and morphological observationThe specimen was collected at a depth of 2 289 m by the Remotely Operated Vehicle Faxian, during the cruise of R/V Kexue in the tropical Western Pacific in June 2019. It was fixed and preserved in 4% buffered formaldehyde for better preservation of morphological details, except that some tissues obtained from fresh material were stored at -80 ℃. The holotype is deposited in the Marine Biological Museum (MBM) of the Chinese Academy of Sciences (CAS) at the Institute of Oceanology, CAS, in Qingdao, China, photographed by Canon EOS 6D camera before fixation. The identification of the holotype is mainly based on typical characters of psychropotid holothurians (Hansen, 1975). Features of external morphology were examined on the basis of images in situ. Small pieces of body tissues (after formaldehyde fixation) were digested in 15% sodium hypochlorite solution to obtain the deposits which were then examined by scanning electron microscope (Hitachi S-3400N).
2.2 PCR amplification and data analysisTotal genomic DNA was extracted from a small pieces of 20–30-mg holothurian muscle tissues (stored at -80 ℃) using OMEGA Mollusc DNA Kit (Omega Biotek Inc., America) according to the manufacture' s guidance. Mitochondrial cytochrome c oxidase I (COI) and 16S rRNA were generated for Benthodytes tetrapapillata sp. nov. using primers and methods as outlined in Miller et al. (2017). PCR amplification program was as follows: initial denaturation for 3 min at 98 ℃, followed by 40 cycles of 10 s at 98 ℃, 10 s at 52 ℃, 10 s at 72 ℃, and a final extension for 5 min at 72 ℃, with a total reaction volume of 50 μL: 1–3-μL template DNA, 25-μL T5 PolStar DNA Polymerase (Tsingke, Beijing, China), 8–10-μL DNase free ddH2O, 2-μL each primer. Then the sequence chromatograms was checked using CHROMAS 2.23 (Technelysium Pty Ltd.). The forward and reverse sequences were assembled by CONTIG EXPRESS (a component of Vector NTI Suite 6.0, Life Technologies, Carlsbad, California).
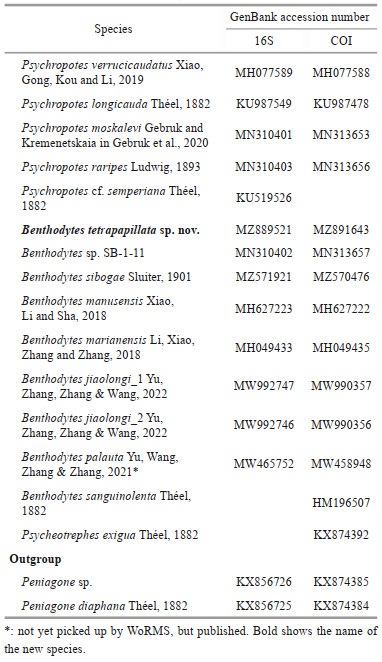
Two partial sequences (COI and 16S) were obtained from holotype of the new species and were deposited in GenBank (for accession numbers see Table 1). In addition, some relevant sequences retrieved from GenBank were used for phylogenetic analyses (Table 1). Two species of genus Peniagone were used to root the tree. Sequences were independently aligned using the MAFFT (Katoh et al., 2019). The alignments were further manually quality checked and corrected based on visual observation. Two genes from the same individual were concatenated into one single alignment (1 255 bp) using software SequenceMatrix v1.8 (Vaidya et al., 2011) for combined analysis.
The maximum likelihood analysis was constructed using RAxMLGUI v1.5 with a rapid bootstrap likelihood analysis (Silvestro and Michalak, 2012), under the GTRGAMMAI substitution model. Bayesian inference (BI) was conducted using the software MrBayes v. 3.1 (Ronquist and Huelsenbeck, 2003) and the optimal DNA substitution model for each partitioned dataset in the BI trees was estimated by the jModelTest 2.1.4, calculated by the Akaike information criterion (AIC) (Darriba et al., 2012), and GTR+G+I model was the most appropriate model for all partitions in the analysis based on a concatenated dataset and single COI gene. The Markov Chain Monte Carlo iterations were run for 1 000 000 generations, with sampling every 100 generations. The first 25% of trees were discarded as burn-in and the remaining trees were summarized in 50% majority rule consensus tree.
3 RESULT 3.1 SystematicsClass Holothuroidea Blainville, 1834
Order Elasipodida Théel, 1882
Family Psychropotidae Théel, 1882
Genus Benthodytes Théel, 1882
Benthodytes tetrapapillata sp. nov. (Figs. 1–4)
|
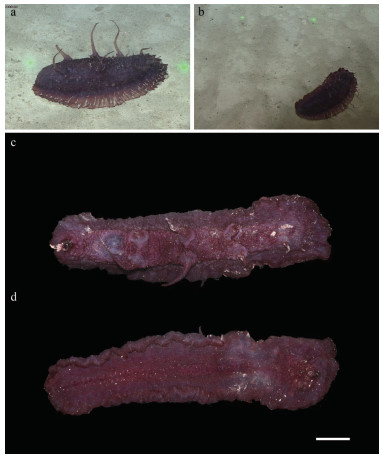
| Fig.1 Benthodytes tetrapapillata sp. nov. (Holotype, MBM286931) a–b. photo of live holotype in situ; c–d. sample fixed with 4% formaldehyde solution. Scale bars: a–b: the distance between laser points is 33 cm; c–d: 3 cm. |
|
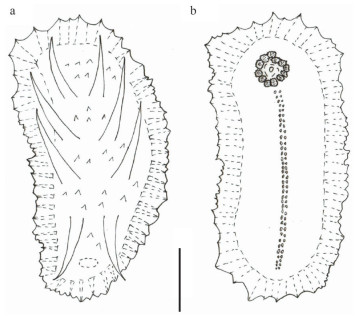
| Fig.2 Drawing of Benthodytes tetrapapillata sp. nov. (Holotype, MBM286931) a. dorsal view; b. ventral view. Scale bar: 7 cm. |
|
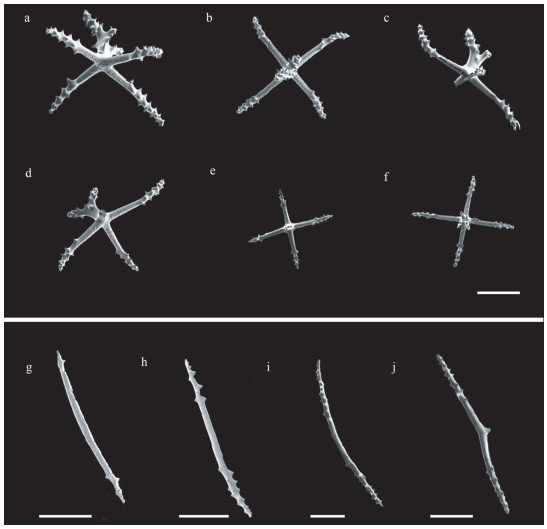
| Fig.3 Scanning electron microscope images of deposits from Benthodytes tetrapapillata sp. nov. MBM286931 a–f. dorsal body wall; g–j. ventral body wall. Scale bar: 200 μm. |
|
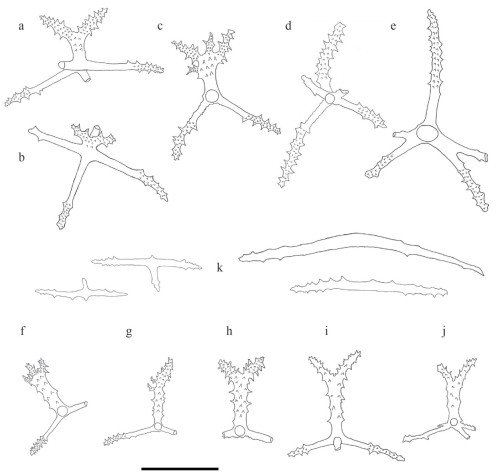
| Fig.4 Drawing of deposits from Benthodytes tetrapapillata sp. nov. a–e. dorsal crosses; f–h. papillae crosses; i–j. tube feet crosses; k. tentacles rods. Scale bar: 200 μm. |
Type material examined
Holotype: 1 adult specimen, catalog number MBM286931, GenBank accession numbers MZ889521, MZ891643, collected on June 9, 2019 from the station FX-Dive 221 (10°02′54″N, 140°08′55″E) on a seamount (named unofficially as "M5") located on the Caroline Ridge in tropical Western Pacific, undersea muddy sediment, 2 289 m.
Type locality
M5 seamount located on Caroline Ridge, tropical Western Pacific Ocean, depth 2 289 m.
Etymology
The Latin word tetrapapillata refers to four pairs of distinct papillae on dorsum.
Diagnosis
Body elongated, dorsum inflated, ventrum flattened, tapered slightly at both ends. Skin dark violet, thick and gelatinous, numerous warts scattered on the surface. Mouth ventral, circum-oral papillae not visible. Tentacle 12. Anus dorsal. Brim broad with clearly visible violet channels. Four pairs of well-developed dorsal papillae, large and conical, tapering to distal extremity, arranged in two single rows along the dorsal radii, evenly distributed. Midventral tube feet present throughout length of the ventral sole. Calcareous ring not visible. Dorsal deposits cross-shaped, arms 200–400 μm long, central apophysis undivided, bipartite, rudimentary, tripartite and tetrapartite. Rods present in ventrum and tentacles, up to 970 μm long.
Description of the holotype
Holotype in situ 32 cm long, 16.5 cm wide (Fig. 1a & b), and about 27 cm in length, 6.5 cm in width after preservation in 4% formaldehyde solution for several days (Fig. 1c–d). Color uniformly dark violet both dorsally and ventrally. Anus dorsal. Skin thick covered with warts, soft and gelatinous. Brim broad and flat, but slight shrinkage occurred after being fixed a few days. Four pairs of dorsal papillae rather big and obvious, arranged in two single rows, distributed regularly along the dorsum, distance between each pair of papillae is approximately the same (Figs. 1a, c, & 2a). The first and fourth pairs are close to the anterior and posterior body ends, respectively; the second and third pairs are larger than the first and fourth pairs, and each papillae in situ is about 10 cm long. Mouth ventral. Tentacles 12, retracted to form the disc which is soft and pliable (Fig. 2b). Midventral tube feet minute and arranged in alternating double row throughout whole length of ventrum (Figs. 1d & 2b). Calcareous ring not visible.
Dorsal deposits crosses with slender and arcuate arms, arms about 200–400 μm in length, bearing many small irregular spines close to tips. Central apophysis with numerous spines, including presence of five main types: (1) undivided and very long, about 170–295 μm (Fig. 4d–e); (2) bipartite, about 130–160 μm in length, usually bifurcating from half position (Figs. 3a–d & 4a–b); (3) rudimentary (Fig. 3e); (4) tripartite (Fig. 3f); (5) tetrapartite, about 174 μm long (Fig. 4c). Deposits of dorsal papillae crosses, central apophysis 156–183 μm in length, containing three types: undivided, bifurcated, and tripartite, bifurcating from a quarter position from the top (Fig. 4f & h). Both ventral and tentacles deposits rod-shaped, with a length of 230–970 μm, slightly curved into two types: (1) small spines at both ends, center smooth (Figs. 3g–i & 4k); (2) an extra bifurcation in the center (Figs. 3j & 4k). Tube feet deposits crosses, with bifurcated central apophysis about 165–217 μm in length (Fig. 4i–j), bearing fewer spines compared to that of dorsal papillae (Fig. 4f & h).
Relationship
Benthodytes tetrapapillata sp. nov. closely resembles Benthodytes gosarsi Gebruk, 2008 in presence of four pairs of evenly distributed dorsal papillae. For external features, the new species differs from B. gosarsi in body color, the former is dark violet (Fig. 1) while the latter is green which is unique among psychropotid holothurians (normally violet). Meanwhile, dorsal papillae of B. tetrapapillata sp. nov. are relatively larger and more developed than those of B. gosarsi. For internal deposits morphology, B. gosarsi has dorsal crosses with a bipartite central apophysis, while B. tetrapapillata sp. nov. has dorsal crosses with varying types of central apophysis (Figs. 3a–f & 4a–e). In the original description, deposits are entirely lacked on ventrum in B. gosarsi, while rods present ventrally in B. tetrapapillata sp. nov. (Fig. 3g–j).
Distribution
Only in the type locality.
3.2 Molecular phylogenetic analysesThe molecular phylogenetic trees (ML and BI) reconstructed based on a total of concatenated data were shown in Fig. 5, and trees based on COI gene were provided in Supplementary Fig.S1. All trees showed that the genus Benthodytes was not monophyletic, as some species of genera Psychropotes and Psycheotrephes were nested within the genus Benthodytes, suggesting the classification of family Psychropotidae need to be redefined. The two consensus trees using ML and BI criteria based on concatenated data were generally congruent (Fig. 5), which showed that Benthodytes tetrapapillata sp. nov. clustered together with Benthodytes manusensis forming a well-supported clade (BS=95, BPP=1), sister to Benthodytes sibogae and Benthodytes palauta (BS=99, BPP=1). The phylogenetic trees inferred using ML and BI criteria based on single gene COI (Supplementary Fig.S1) showed similar overall topology. These results support the separation of Benthodytes tetrapapillata sp. nov. from other congeners.

|
| Fig.5 Phylogenetic trees of Psychropotidae based on a concatenated dataset of 16S and COI genes The topology follows the result of BI tree, numbers at each node are ML analysis bootstrap values (BS) and Bayesian posterior probabilities (BPP). Nodes marked by black dots are supported by ML and BI trees. Red font means new species. |
State of main morphological characters in valid species of Benthodytes is provided in Table 2. Benthodytes was divided into two distinct groups based on the details of external and deposits morphology (Hansen, 1975; Yu et al., 2022). Benthodytes tetrapapillata sp. nov. belongs to the group characterized by cross-shaped deposits and well-developed dorsal papillae. The morphological identification was supported by molecular evidence. The phylogenetic trees (Fig. 5; Supplementary Fig. S1) revealed a congruent topology of Benthodytes, which is divided into two clades, verifying that Benthodytes tetrapapillata sp. nov. is a valid species within the Benthodytes lineage.
Phylogenetic analyses indicated that the new species was closely related to Benthodytes manusensis, with high support (16S-COI tree: BS=95, BPP=1; COI tree: BS=94, BPP=1). From a morphological point of view, both B. manusensis and the new species have 12 tentacles, big and conspicuous papillae which arrange in two single rows and evenly distributed (Table 2). In deposits morphology, the two species both have primary crosses with four arms and a central apophysis on dorsum (Table 2). However, the new species differs from B. manusensis in the number and position of dorsal papillae. B. tetrapapillata sp. nov. has four pairs of papillae, while B. manusensis only has three pairs. The position of the first pair of large papillae of B. tetrapapillata sp. nov. is approximately in the front quarter of the body (Fig. 2b), while that of B. manusensis is very close to the anterior end which is really unique among psychropotid species. Moreover, for dorsal deposits, B. tetrapapillata sp. nov. has crosses with undivided, rudimentary, tripartite and tetrapartite apophysis (Figs. 3e, f, & 4c–e) besides the bipartite apophysis of B. manusensis. On ventrum, B. tetrapapillata sp. nov. has rods (Fig. 3g–j) while B. manusensis has smaller crosses.
The molecular phylogenetic trees (Fig. 5; Supplementary Fig.S1) of the family Psychropotidae appear to be topologically similar and well in agreement with previous studies (Li et al., 2018; Xiao et al., 2018; Yu et al., 2021). Benthodytes was divided into two clades: Benthodytes sanguinolenta and B. jiaolongi grouped in one clade; B. marianensis, B. sp. SB-1-11, B. manusensis, B. sibogae, B. palauta, and the new species grouped in the other one clade. Since two groups also show great differences in morphological characters: the species in former group are characterized by mostly reduced rod deposits and minute dorsal papillae, while the species in later group are characterized by the regular crosses and well-developed dorsal papillae, it suggests that the species in former group may form a new genus under the family Psychropotidae, but the hypothesis cannot be verified due to the limited number of molecular data and specimens, and more studies need to be supplemented for a more complete taxonomic system. What' s more, further sequence data of Psychropotidae is needed to resolve the placement of B. tetrapapillata sp. nov. relative to other Benthodytes species. Sequences obtained in this study will enrich the database and provide more data to clarify the position of the genus Benthodytes in the taxonomy.
5 CONCLUSIONPhylogenetic analyses revealed the separation of Benthodytes tetrapapillata sp. nov. from congeners of genus Benthodytes, and supported the morphological identification that Benthodytes tetrapapillata sp. nov. was a new psychropotid species. The new species is characterized by four pairs of rather large and evenly distributed papillae, and crosses with varying types of central apophysis. Benthodytes tetrapapillata sp. nov. is the first species of order Elasipodida reported on a seamount (M5) located on the Caroline Ridge. The information about this new species can be significantly useful in expanding the diversity of the psychropotid species in deep-sea seamounts.
6 DATA AVAILABILITY STATEMENTThe data generated and analyzed to support the research result can be provided by the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTThe authors are extremely grateful to the assistance of the crew of ROV Faxian for sample collection and in-situ pictures shooting. Many thanks to the members of Department of Marine Organism Taxonomy & Phylogeny at the Institute of Oceanology, CAS (IOCAS) for their help in sample collection and analysis. We are grateful to three referees for their constructive comments.
Electronic supplementary material
Supplementary material (Supplementary Fig.S1) is available in the online version of this article at https://doi.org/10.1007/s00343-022-2220-0.
Billett D S M. 1988. The Ecology of Deep-Sea Holothurians. University of Southampton, Southampton.
|
Clark M R, Rowden A A, Schlacher T, et al. 2010. The ecology of seamounts: structure, function, and human impacts. Annual Review of Marine Science, 2: 253-278.
DOI:10.1146/annurev-marine-120308-081109 |
Darriba D, Taboada G L, Doallo R, et al. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9(8): 772.
DOI:10.1038/nmeth.2109 |
Dower J F, Mackas D L. 1996. "Seamount effects" in the zooplankton community near Cobb Seamount. Deep Sea Research Part Ⅰ: Oceanographic Research Papers, 43(6): 837-858.
DOI:10.1016/0967-0637(96)00040-4 |
Gebruk A V. 1990. Deep-Sea Holothurians of the Family Elpidiidae. Nauka, Moscow. 160p.
|
Gebruk A V. 2008. Holothurians (Holothuroidea, Echinodermata) of the northern Mid-Atlantic Ridge collected by the G. O. Sars MAR-ECO expedition with descriptions of four new species. Marine Biology Research, 4(1-2): 48-60.
DOI:10.1080/17451000701842898 |
Gebruk A V, Budaeva N E, King N J. 2010. Bathyal benthic fauna of the Mid-Atlantic Ridge between the Azores and the Reykjanes Ridge. Journal of the Marine Biological Association of the United Kingdom, 90(1): 1-14.
DOI:10.1017/S0025315409991111 |
Genin A. 2004. Bio-physical coupling in the formation of zooplankton and fish aggregations over abrupt topographies. Journal of Marine Systems, 50(1-2): 3-20.
DOI:10.1016/j.jmarsys.2003.10.008 |
Hansen B. 1975. Scientific results of the Danish Deep-Sea Expedition Round the World 1950-52. Systematics and biology of the deep-sea holothurians. Part 1: elasipoda. Galathea Report, 13: 1-262.
|
Katoh K, Rozewicki J, Yamada K D. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20(4): 1160-1166.
DOI:10.1093/bib/bbx108 |
Koehler R, Vaney C. 1905. An Account of the Deep-Sea Holothuroidea Collected by the Royal Indian Marine Survey Ship Investigator. The Indian Museum, Calcutta. 170p.
|
Li Y N, Xiao N, Zhang L P, et al. 2018. Benthodytes marianensis, a new species of abyssal elasipodid sea cucumbers (Elasipodida: Psychropotidae) from the Mariana Trench area. Zootaxa, 4462(3): 443-450.
DOI:10.11646/zootaxa.4462.3.10 |
Ludwig H. 1893. Vorläufiger bericht über die erbeuteten holothurien. In: Reports on the Dredging Operations off the West Coast of Central America to the Galapagos, etc., by the U.S. Fish Commission Steamer "Albatross". IV. Bulletin of the Museum of Comparative Zoöology at Harvard College. 24(4): 105-114.
|
Ludwig H L. 1894. The Holothurioidea. Reports on an exploration off the west coasts of Mexico, Central and South America, and off the Galapagos Islands, in charge of Alexander Agassiz, by the U. S. Fish Comission Streamer "Albatross", during 1891. Memoirs of the Museum of Comparative Zoology at Harvard College, 17(3): 1-183.
|
Martinez M I, Solís-Marín F A, Penchaszadeh P E. 2014. Benthodytes violeta, a new species of a deep-sea holothuroid (Elasipodida: Psychropotidae) from Mar del Plata Canyon (south-western Atlantic Ocean). Zootaxa, 3760(1): 89-95.
DOI:10.11646/zootaxa.3760.1.6 |
Miller A K, Kerr A M, Paulay G, et al. 2017. Molecular phylogeny of extant Holothuroidea (Echinodermata). Molecular Phylogenetics and Evolution, 111: 110-131.
DOI:10.1016/j.ympev.2017.02.014 |
Paulay G, Rogacheva A. 2022. World list of holothuroidea. Catalogue of Life Checklist, March 2022, https://doi.org/10.48580/dfpd-3c7. Accessed on 2022-03-21.
|
Perrier R. 1896. Sur les Elasipodes recueillis par le Travailleur et le Talisman. Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 123: 900-903.
|
Rogacheva A, Cross I A, Billett D S M. 2009. Psychropotid holothurians (Echinodermata: Holothuroidea: Elasipodida) collected at abyssal depths from around the Crozet Plateau in the Southern Indian Ocean. Zootaxa, 2096(1): 460-478.
DOI:10.11646/zootaxa.2096.1.28 |
Rogacheva A, Gebruk A, Alt C H S. 2013. Holothuroidea of the Charlie Gibbs fracture zone area, northern mid-Atlantic ridge. Marine Biology Research, 9(5-6): 587-623.
DOI:10.1080/17451000.2012.750428 |
Ronquist F, Huelsenbeck J P. 2003. MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics, 19(12): 1572-1574.
DOI:10.1093/bioinformatics/btg180 |
Silvestro D, Michalak I. 2012. raxmlGUI: a graphical frontend for RAxML. Organisms Diversity & Evolution, 12(4): 335-337.
DOI:10.1007/s13127-011-0056-0 |
Sluiter C P. 1901a. Die Holothurien der Siboga-Expedition. Biodiversity Heritage Library OAI Repository. 44: 1-142. (in German)
|
Sluiter C P. 1901b. Neue holothurien aus der Tiefsee des Indischen Archipels Gesammelt durch die Siboga-Expedition. Tijdschrift der Nederlandsche Dierkundige Vereeniging, 7(1): 1-28.
|
Théel H. 1882. Report on the Holothuroidea. Part I. Report of the scientific results of the voyage of H.M.S. Challenger. Zoology, 4(13): 1-176.
|
Tyler P A, Rice A L, Young C M et al. 1996. A walk on the deep side: animals in the deep sea. In: Thorpe S A ed. Oceanography: An Illustrated Guide. CRC Press, London. p. 195-211.
|
Vaidya G, Lohman D J, Meier R. 2011. SequenceMatrix: concatenation software for the fast assembly of multigene datasets with character set and codon information. Cladistics, 27(2): 171-180.
DOI:10.1111/j.1096-0031.2010.00329.x |
WoRMS. 2022. Benthodytes Théel, 1882. https://www.marinespecies.org/aphia.php?p=taxdetails&id=123529. Accessed on 2022-03-24.
|
Xiao N, Li X M, Sha Z L. 2018. Psychropotid holothurians (Echinodermata: Holothuroidea: Elasipodida) of the tropical Western Pacific collected by the KEXUE expedition with description of one new species. Marine Biology Research, 14(8): 816-826.
DOI:10.1080/17451000.2018.1546012 |
Xiao Y L, Xiao N, Zeng X Q. 2020. New records of a genus and a species of Psychropotidae (Echinodermata: Holothuroidea: Elasipodida) from the South China Sea. Oceanologia et Limnologia Sinica, 51(3): 644-648.
(in Chinese with English abstract) DOI:10.11693/hyhz20191200283 |
Yang H S, Xiao N, Zhang T. 2016. Present status and prospect of the study of echinoderms. Studia Marina Sinica, (51): 125-131.
(in Chinese with English abstract) DOI:10.12036/hykxjk20160719004 |
Yesson C, Clark M R, Taylor M L, et al. 2011. The global distribution of seamounts based on 30 arc seconds bathymetry data. Deep Sea Research Part Ⅰ: Oceanographic Research Papers, 58(4): 442-453.
DOI:10.1016/j.dsr.2011.02.004 |
Yu C, Wang C S, Zhang D S, et al. 2021. Benthodytes palauta, a new species of deep-sea holothuroid (Elasipodida: Psychropotidae) from the western Pacific Ocean. Acta Oceanologica Sinica, 40(12): 50-54.
DOI:10.1007/s13131-021-1937-5 |
Yu C, Zhang D S, Zhang R Y, et al. 2022. New psychropotid species (Echinodermata, Holothuroidea, Elasipodida) of the Western Pacific with phylogenetic analyses. ZooKeys, 1088: 99-114.
DOI:10.3897/zookeys.1088.69141 |
 2023, Vol. 41
2023, Vol. 41