Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HUANG Yuhuan, SUN Chengjun, LÜ Lina, DING Neal Xiangyu, YU Liangmin, YANG Guipeng, DING Haibing
- Characteristics of vertical distributions of methane and dimethylsulphoniopropionate in the southern Yap Trench
- Journal of Oceanology and Limnology, 41(6): 2101-2116
- http://dx.doi.org/10.1007/s00343-022-2063-8
Article History
- Received Feb. 9, 2022
- accepted in principle May 5, 2022
- accepted for publication Sep. 8, 2022
2 Marine Ecology and Environmental Science Laboratory, Laoshan Laboratory (Qingdao), Qingdao 266237, China;
3 Marine Bioresource and Environment Research Center, the First Institute of Oceanography, Ministry of Natural Resources, Qingdao 266061, China;
4 College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China;
5 Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China;
6 College of Agriculture and Life Sciences, Cornell University, Ithaca, NY 14853, USA
As the most abundant organic gas in the atmosphere, methane (CH4) plays a direct role in greenhouse effect on the earth and the global carbon cycle (Valentine, 2011; IPCC, 2013). CH4 affects global climate change, atmospheric pollution, and carbon biogeochemical cycle in the ocean significantly. Ocean is an important CH4 source for the atmosphere (Reeburgh, 2007). Despite methanogenesis requires strictly anaerobic conditions, pelagic CH4 supersaturation frequently occurs in oxic surface seawater in the open ocean. This phenomenon has been termed the "Oceanic Methane Paradox", and the interest in studying the paradox has been surging in recent years (Tilbrook and Karl, 1995; Lenhart et al., 2016). In addition to greenhouse gases such as CH4 and carbon dioxide (CO2), anti-greenhouse gas, dimethyl sulfur (DMS), as a climate-active volatile gas in the atmosphere, also plays important role in controlling global climate change. Ocean is the most important source of the atmosphere DMS (Kettle and Andreae, 2000). Dimethylsulphoniopropionate (DMSP) as a precursor of DMS (Yoch, 2002), is produced by marine algae, corals, plants, and heterotrophic bacteria (Zheng et al., 2020). In these organisms, DMSP is proposed to act as an osmolyte, cryoprotectant, predator deterrent, and/or antioxidant. Considering DMSP is a key source of carbon, reduced sulfur, and/or energy for microbial communities (Kiene et al., 2000), it plays an essential role in the marine ecosystem. Series of studies have explored the relationships between CH4 and DMSP in different marine environments, and DMS and DMSP have been found as precursors of CH4 in recent studies (Damm et al., 2008, 2010; Florez-Leiva et al., 2013; Zindler et al., 2013; Zhai et al., 2019). However, few studies investigated their relationships in deep sea regions until now.
Abyssal layer (4 000–6 000 m) and hadal zone (6 000–11 000 m), as extreme environments with aphotic and high-pressure, provide ideal places for studying biogeochemical processes of CH4 and DMSP from sea surface to deep ocean. Due to the difficulty of reaching and sampling, the studies on the distributions of CH4 and DMSP are absent in the abyssal layer and the hadal zone. In recent years, the advances of manned submersibles and deep-sea technology thereby stimulate a wave of hadal exploration. The vertical profiles of DMSP in the Challenger Deep of the Mariana Trench have been reported in March 2017 (Zheng et al., 2020). Zhang et al. (2018) documented the vertical distributions of CH4 and DMSP in the water column of the northern Yap Trench in May 2016. However, limited studies were conducted on the biogeochemistry of CH4 and DMSP in the marine trench area until now.
The Yap Trench is adjacent to the southern end of the Mariana Trench and links to the northern end of the Palau Trench (Beccaluva et al., 1980) (Fig. 1). It connects the waters in the Mariana-Caroline basins, playing important role in the arc-trench system of the northwest Pacific Ocean (Kaneko et al., 1998). The trench is approximately 700 km long and 50 km wide with the deepest point of 8 527 m (Lee, 2004). It is divided into the northern and the southern parts at 8.43°N by a sill (Fujiwara et al., 2000). The northern Yap Trench is located at the junction between the Oceanic Pacific Plate, the Philippine Plate, and the Caroline Plate. As a comparison, the southern Yap Trench is between two plates: the Philippine Plate and the Caroline Plate (Ding and Sun, 2020). The trench thus has significantly different hydrodynamic characteristics between its northern and southern regions, making it a unique area in studying the marine biogeochemical process of CH4 and DMSP in deep sea environment.
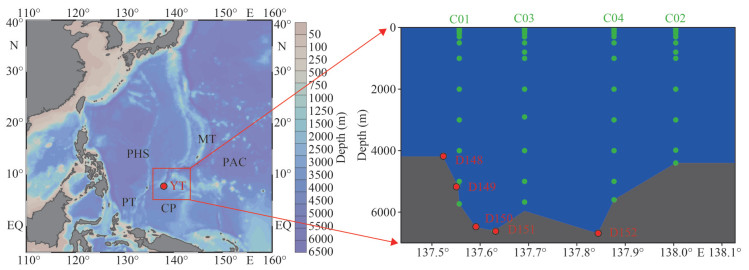
|
| Fig.1 Study area and location of sampling stations in the southern Yap Trench, including four stations from water columns (C01, C02, C03, C04) and five stations at Benthic Boundary Layer (D148, D149, D150, D151, D152) YT: Yap Trench; MT: Mariana Trench; PT: Palau Trench; PHS: Philippine Sea Plate; PAC: Pacific Plate; CP: Caroline Plate. |
In this study, we investigated the vertical distribution characteristics of CH4 and DMSP from sea surface to the hadal zone in the southern Yap Trench. The specific objectives of this study were to (1) describe the vertical profiles of CH4 and DMSP in the water column and the Benthic Boundary Layer (BBL); (2) attempt to elaborate the source of elevated concentrations of DMSP in the abyssal layer; (3) elucidate if the sediment is a source of CH4 and DMSP for the water column; and (4) explore the relationships between CH4 and DMSP from surface water to bottom water and try to explain CH4 surplus in the euphotic layer. The results of this study would expand our understanding of the biogeochemical processes of CH4 and DMSP from the sea surface to the hadal zone.
2 MATERIAL AND METHOD 2.1 Study areaThe Yap Trench abyssal water marked by cold, high salinity, and high oxygen concentration originates from the East Mariana Basin (EMB) and the East Caroline Basin (ECB), as a part of the western propagating Lower Circumpolar Deep Water (LCPW) (Liu et al., 2018, 2020). The current is northward in its northern region. Whilst, in the southern region, it has the southward flow in the west and the northward flow in the east, and the net transport is southward (Johnson and Toole, 1993; Kawabe and Taira, 1998; Kawabe et al., 2003; Liu et al., 2018, 2020). The circulation of LCPW is impeded by sills in trenches and basins, and the water cannot reach below 6 000 m. Therefore, the hadal water seems to be of the isolated local water rather than LCPW (Liu et al., 2020).
2.2 Sample collection and processingThe R/V Xiangyanghong 09 carried the deep-sea manned Jiaolong submersible conducted Voyage 38 cruise of the China Ocean Mineral Resources Research and Development Association in the southern Yap Trench in June 2017. The topography of the study area and the sampling stations are shown in Fig. 1. The specific information is shown in Supplementary Table S1. Seawater samples from the water column were collected using 12-L Niskin bottles attached to an SBE 911 plus conductivity-temperature-depth (CTD) system (Seabird electronics, USA), which CTD rosette can directly obtain samples below 6 000 m. Five seawater samples from the BBL were obtained by Jiaolong submersible with pressure-tight and air-tight seawater sampler (Fig. 1).
Seawater samples for CH4 analysis were immediately collected after CTD equipment was recovered and the submersible returned to the vessel. 310-mL flasks were rinsed previously with methanol, dichloromethane, n-hexane, and combusted at 450 ℃ for 6 h in a muffle furnace. A rubber-connecting tube was inserted into the bottom of the flask and the seawater sample was slowly injected. The flask was rinsed 3 times with the seawater. After overflowing about 2-fold of the flask volume, 1.0-mL saturated solution of mercuric chloride (HgCl2) was added to inhibit microbial activity. The sample flask was immediately sealed with a butyl rubber stopper and aluminum cap. To avoid atmospheric contamination, no bubbles appeared when the seawater was injected into the flask. Samples were stored at 4 ℃ upsides down in the dark and analyzed after returning to land laboratory (Zhang et al., 2008).
For dissolved dimethylsulphoniopropionate (DMSPd) analysis, 8-mL seawater sample was filtered into a 10-mL centrifuge tube by gravity filtration protocol with Whatman GF/F glass fiber filter (diameter 47 mm). Another 8-mL seawater for total dimethylsulphoniopropionate (DMSPt) analysis was directly transferred into a 10-mL centrifuge tube. To remove DMS in seawater, about 80-μL 50% H2SO4 was added to the sample to pH < 2. Samples were stored at 4 ℃ in dark until analysis (Kiene and Slezak, 2006).
2.3 Sample analysisConcentrations of CH4 were measured using static headspace equilibration method (Jayakumar et al., 2001; Li et al., 2017). The 50-mL space was created by replacing seawater with ultra-pure nitrogen (N2) in the 310-mL flask. The flask was shaken vigorously for two minutes and equilibrated for 12 h in the dark. The 1-mL gas was drawn from the headspace using a gastight glass syringe and was injected into gas chromatography (GC-2014, Shimadzu Company). CH4 was separated by a stainless-steel column (3.66 m×3.18 mm×2 mm, n-Octane on Res-SilC, Restek, USA) and detected by flame ionization detector (FID). The temperature of the oven, the injector, and the detector were 40, 100, and 175 ℃, respectively. The carrier gas (N2) flow velocity was 10 mL/min. The concentrations were calibrated with standard CH4 gas (1.98×10-6, 3.95× 10-6, 6×10-6, 8×10-6, and 10×10-6, the Research Institute of China National Materials). The detection limit of this method was 0.08 nmol/L, and the precision of repeated analyses was about ±5%. The saturation (R, %) of CH4 was calculated by the following equations as
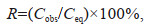
where Cobs is the observed concentration of CH4 (nmol/L), Ceq is the equilibrium concentration of CH4 with ambient atmospheric concentration (1.843×10-6) (https://www.esrl.noaa.gov/gmd). Ceq is calculated based on the ambient atmospheric concentration of CH4, in-situ temperature, and salinity of seawater (Wiesenburg and Guinasso, 1979).
Concentrations of DMSPd and DMSPt were obtained indirectly by measuring concentrations of their transformed compound—DMS (Dacey and Blough, 1987). The 2-mL seawater sample was transferred into a 10-mL vial and then sealed with a butyl rubber stopper and aluminum cap. To ensure DMSP be completely converted to DMS, seawater sample was treated with 200 μL of 10-mol/L KOH solution and stored in the dark (4 ℃) for more than 24 h. The high-purity nitrogen gas was used to sweep DMS at room temperature. The gas was collected at a cold trap. After the extraction, the adsorption cold trap was heated in a hot water bath and the trapped gas was desorbed and injected into GC (Agilent Technologies, Palo Alto, CA, USA) equipped with 10% DEGS/Chromosorb W-AW-DMCS 60–80 mesh column and detected by a flame photometric detector (FPD). The precision of this method was approximately 5% and the detection limit was 0.30 nmol/L (Yang et al., 2011; Zhai et al., 2019). Concentrations of particle dimethylsulphoniopropionate (DMSPp) in seawater samples were the difference between the concentrations of DMSPt and DMSPd.
Data of temperature (T), salinity (S), and concentrations of dissolved oxygen (DO) were obtained from CTD profiles and dissolved oxygen sensors. Concentrations of chlorophyll a (Chl a) and nutrients were obtained from the Second Institute of Oceanography, Ministry of Natural Resource, China.
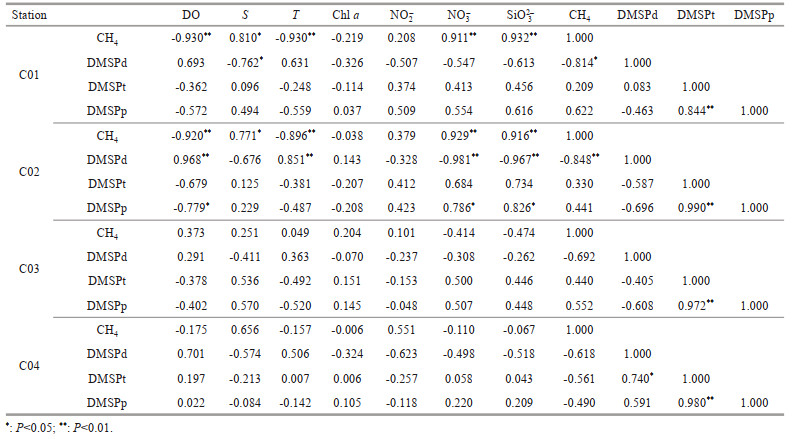
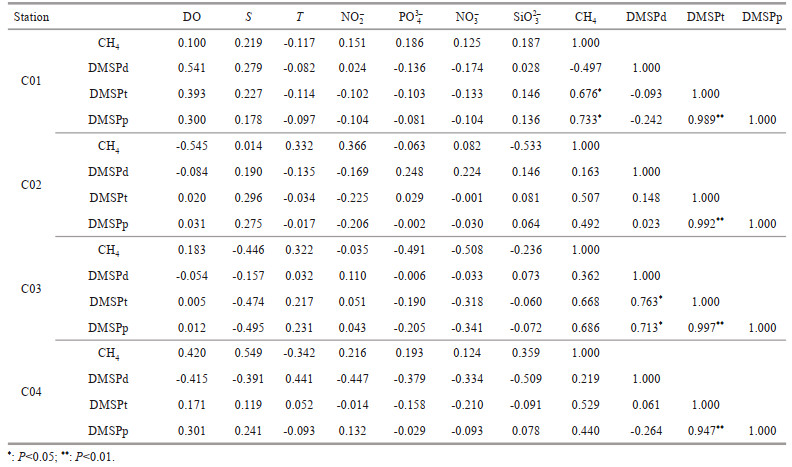
2.4 Statistical analysisThe correlations among concentrations of CH4, DMSPd, DMSPp, DMSPt, DO, Chl a, NO2–, NO3–, SiO32-, and temperature and salinity in the seawater of the southern Yap Trench were examined using Pearson's correlation analysis, which was conducted in SPSS version 22 (IBM, U. S. A.). The Pearson's correlation coefficients were used to establish a matrix of correlations to estimate the possible relationships between CH4, DMSPd, DMSPp, DMSPt, DO, Chl a, NO2–, NO3–, SiO32–, temperature, and salinity in the seawater. Analysis of Variance (ANOVA) was conducted by SPSS to test the significance of the mean difference of various parameters between different water layers.
3 RESULTConcentrations of CH4, DMSPd, DMSPp, and DMSPt in the seawater samples from the water columns (n=4) and the BBL (n=5) in the southern Yap Trench were measured. To clearly describe vertical profiles of these parameters, we tracked their variation in five different layers separately, including the euphotic layer (0–200 m), the mesopelagic layer (200–1 000 m), the bathypelagic layer (1 000–4 000 m), the abyssal layer (4 000–6 000 m), and the hadal zone (below 6 000 m).
3.1 Hydrological conditionThe temperature, salinity, and concentrations of DO in the water columns are shown in Table 1. In the study area, seawater temperature decreased with depth. The highest temperature (C03, 0 m) and the lowest temperature (C02, 4 400 m) were 29.90 and 1.48 ℃, respectively, with a thermocline at 75–150 m. Seawater salinity varied from 33.33 to 34.85 (minimum at C04 (0 m) and maximum at C04 (100 m)), and a halocline also appeared at 75–150 m. In the four water columns, concentrations of DO sharply decreased from surface water to 200-m depth but reached consistent levels in 200–800 m and then increased slightly with depth. The values ranged 58.65–212.02 μmol/L (lowest at C02 (200 m) and highest at C04 (50 m)), and the minimum value occurred in 200–1 000 m in all water columns.

|
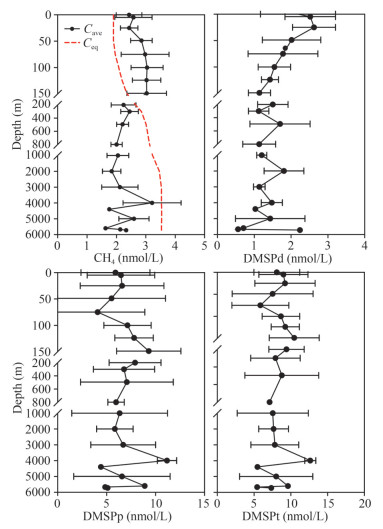
Vertical profiles of CH4 concentrations in the water column are shown in Figs. 2–3. In general, concentrations of CH4 increased with depth in the euphotic layer and decreased at a consistent level below 200 m in all the stations. An exception occurred around 4 000-m depth where concentrations of CH4 increased significantly. Concentrations of CH4 ranged 1.5–4.5 nmol/L (minimum at C03 (2 000 m) and maximum at C04 (4 000 m)). The highest concentrations were 3.9 (C01), 3.0 (C02), 3.3 (C03), and 4.5 nmol/L (C04) in the four water columns at 150, 150, 4 000, and 4 000 m, respectively. In the euphotic layer, the maximum layer of concentrations of CH4 was consistent with the thermocline and the halocline around 75–150 m, and the highest (C04, 75 m) and the lowest values (C03, 0 m) were 4.0 and 2.0 nmol/L, respectively. The mean concentrations of CH4 were 2.8±0.6, 2.3±0.3, 2.0±0.4, and 2.6±0.8 nmol/L in the euphotic layer, the mesopelagic layer, the bathypelagic layer, and the abyssal layer, respectively. Based on ANOVA, the mean concentration of CH4 in the euphotic layer was higher than those in other water layers. Concentrations of CH4 were supersaturated with equilibrium concentrations ranging from 94% to 204% in the euphotic layer (Fig. 3). Hence, surface water of the Yap Trench is a CH4 source for the ambient atmosphere.
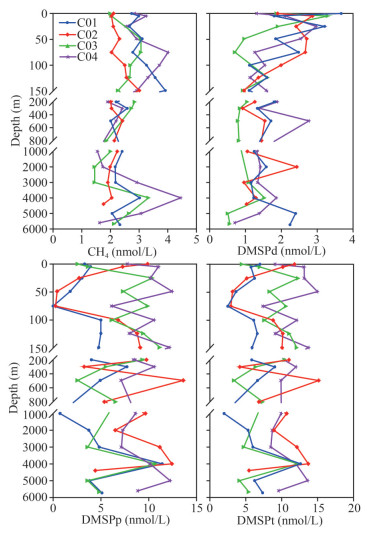
|
| Fig.2 Vertical profiles of concentrations of CH4, dissolved DMSP (DMSPd), particulate DMSP (DMSPp), and total DMSP (DMSPt) in the seawater of the southern Yap Trench |
|
| Fig.3 Vertical profiles of average concentrations of CH4, dissolved DMSP (DMSPd), particulate DMSP (DMSPp), and total DMSP (DMSPt) in the seawater of the southern Yap Trench Black dots denoting average concentration and red dotted line indicating CH4 equilibrium concentration. |
Concentrations of CH4 changed insignificantly in 200–3 000-m depth (Figs. 2–3). The values ranged from 1.9 to 2.9 nmol/L in the mesopelagic layer with the minimum and the maximum at C03 (800 m) and C03 (200 m), respectively. The concentrations varied from 1.5 to 2.9 nmol/L in the bathypelagic layer with the lowest value and the highest value at C03 (2 000 m) and C04 (3 000 m), respectively. It had an exception around 4 000 m where CH4 concentrations increased prominently. Below 4 000-m depth, concentrations of CH4 decreased rapidly with the maximum (4.5 nmol/L) and the minimum (1.6 nmol/L) at station C04 (4 000 m) and station C04 (5 600 m) in the abyssal layer, respectively.
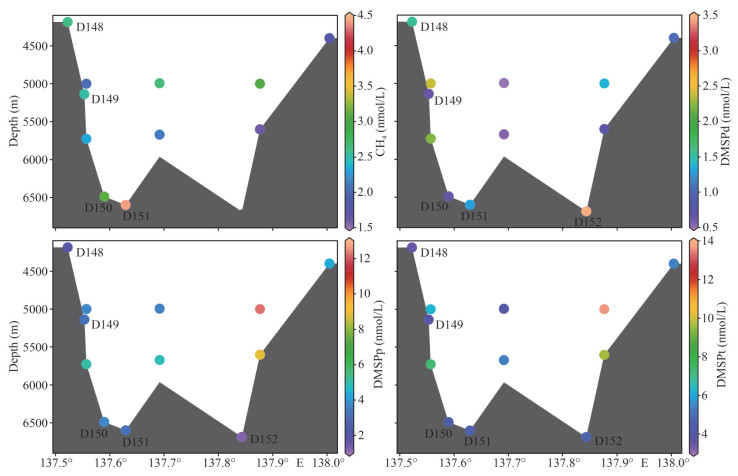
3.2.2 Distribution of CH4 in the seawater of the Benthic Boundary LayerIn the seawater of the Benthic Boundary Layer, concentrations of CH4 ranged 2.5–4.4 nmol/L (minimum at station D149 (5 136 m) and maximum at station D151 (6 599 m)) (Fig. 4). The mean concentration was 3.2±0.9 nmol/L, being slightly higher than those values in other layers of the water column. In addition, concentrations of CH4 in the seawater of the Benthic Boundary Layer were slightly higher than those in the water column at a similar depth (Fig. 4). Therefore, the sediment should be a weak CH4 source for the water column in the southern Yap Trench. The enrichment of organic matter in the sediment of the trench by funnel effect provided a proper condition for methanogenesis and then promoted the production of CH4.
|
| Fig.4 Distributions of concentrations of CH4, dissolved DMSP (DMSPd), particulate DMSP (DMSPp), and total DMSP (DMSPt) in the seawater of the Benthic Boundary Layer in the southern Yap Trench |
Vertical profiles of concentrations of DMSPd, DMSPp, and DMSPt in the water columns are shown in Figs. 2–3. Concentrations of DMSPd decreased sharply with depth in the euphotic layer and maintained at consistent levels below 200 m. The concentrations ranged 0.5–3.7 nmol/L (lowest at station C03 (5 000 m) and the highest appeared at station C01 (0 m)). In the four water columns, the highest concentrations were 3.7 nmol/L (station C01, 0 m), 2.9 nmol/L (station C02, 5 m), 3.3 nmol/L (station C03, 3 m), and 3.0 nmol/L (station C04, 25 m). Obviously, the highest concentration of DMSPd occurred at the surface layer. In the euphotic layer, concentrations of DMSPd ranged 0.9–3.7 nmol/L (minimum at station C03 (150 m) and reached maximum at station C01 (0 m)). The mean DMSPd concentration in the euphotic layer was 1.9±0.8 nmol/L, being slightly higher than 1.4±0.5, 1.4±0.4, and 1.3±0.6 nmol/L in the mesopelagic layer, bathypelagic layer, and abyssal layer, respectively.
Concentrations of DMSPp had an increasing trend in the euphotic layer and decreased inconspicuously in 200–3 000 m (Figs. 2–3). Below 3 000-m depth, concentrations of DMSPp had a notably increasing trend at 4 000-m depth and decreased below it. The concentrations varied from 0 to 13.6 nmol/L (minimum at C01 (75 m) and maximum at C02 (500 m)). In the four water columns, the highest concentrations of DMSPp were 11.4 nmol/L (station C01, 4 000 m), 13.6 nmol/L (station C02, 500 m), 11.2 nmol/L (station C03, 150 m), and 12.5 nmol/L (station C04, 50 m). In the euphotic layer, the average concentration of DMSPp increased from sea surface to 150-m depth and then slightly decreased with depth to 200 m. The depth with the maximum concentration of DMSPp was consistent with the thermocline and the halocline in the euphotic layer (Table 1). The mean concentration of DMSPp were 6.6±3.7, 7.1±3.1, 6.3±3.1, and 8.0±3.6 nmol/L in the euphotic layer, the mesopelagic layer, the bathypelagic layer, and the abyssal layer, respectively. The average concentration of DMSPp around 4 000-m depth was highest in the water column, up to 11.2±1.0 nmol/L.
Vertical profiles of concentrations of DMSPt and DMSPp shared similar variation patterns (Figs. 2–3). Concentrations of DMSPt ranged 2.0–15.2 nmol/L (lowest at station C01 (1 000 m) and reached the highest at station C02 (500 m)). In the four water columns, the maxima of concentrations of DMSPt were 12.6 (station C01, 4 000 m), 15.2 (station C02, 500 m), 12.3 (station C03, 25 m), and 15.0 nmol/L (station C04, 50 m). In the euphotic layer, concentrations of DMSPt increased from surface water to 150 m and then slightly decreased with depth to 200 m. The layer with the maxima of concentrations of DMSPt was consistent with the thermocline and the halocline in the euphotic layer. The mean concentrations of DMSPt were 8.5±3.4, 8.5±3.2, 7.7±3.1, and 9.3±3.7 nmol/L in the euphotic layer, mesopelagic layer, bathypelagic layer, and abyssal layer, respectively. The average concentration of DMSPt at 4 000-m depth was the highest in the water column, up to 12.7±0.8 nmol/L.
3.3.2 Distribution of DMSP in the seawater of the Benthic Boundary LayerThe profiles of DMSPd, DMSPp, and DMSPt in the seawater of the Benthic Boundary Layer are shown in Fig. 4. Concentrations of DMSPd ranged 0.6–3.5 nmol/L (minimum at station D149 (5 136 m) and reached maximum at station D152 (6 687 m)). Concentrations of DMSPd in the seawater of the Benthic Boundary Layer were inconspicuously higher than those in the adjacent water column (Fig. 4). In the five Benthic Boundary Layer samples, concentrations of DMSPp ranged 1.2–3.8 nmol/L (minimum at station D152 (6 687 m) and reached maximum at station D150 (6 487 m)). Concentrations of DMSPt varied from 3.6 to 4.7 nmol/L (minimum at station D148 (4 186 m) and reached maximum at station D152 (6 687 m)). The mean concentrations of DMSPp and DMSPt were 2.8±1.1 and 4.3±0.5 nmol/L, respectively. Concentrations of DMSPp and DMSPt in the seawater of the Benthic Boundary Layer were not higher than those in the water column at the similar depth.
4 DISCUSSION 4.1 Heterotrophic bacteria as important DMSP producers in deepwater of the southern Yap TrenchConcentrations of DMSPp and DMSPt shared similar vertical profiles (Figs. 2–3). The concentrations had similar increasing trend in the euphotic layer and maintained at consistent levels between 200–3 000 m. In the euphotic layer, concentrations of DMSPp ranged 0–12.5 nmol/L on average of 6.6±3.7 nmol/L, and concentrations of DMSPt varied from 2.5 to 15.0 nmol/L on average of 8.5±3.4 nmol/L. Concentrations of DMSPp and DMSPt in this study are similar to previous studies (Yang et al., 2011; Zhai et al., 2018; Zheng et al., 2020). For example, Cui et al. (2015) documented that the average concentrations of DMSPp and DMSPt were 7.1±1.5 and 7.9±1.4 nmol/L respectively in the surface seawater of the North Pacific Ocean. In the euphotic layer, the maxima of concentrations of DMSPp and DMSPt occurred at 150-m depth, coinciding with the thermocline and the halocline in the southern Yap Trench (Table 1). DMSP is produced mainly by marine phytoplankton including dinoflagellates, coccolithophorids etc. in the euphotic layer (Zhang et al., 2019). Both phytoplankton biomass and phytoplankton community structure could affect concentrations and existential forms of DMSP in seawater. Thus, it is reasonable to find concentrations of DMSP at higher levels in the euphotic layer.
The synthesis of DMSP in marine phytoplankton cells is periodic and proportional to light intensity and illumination time (Karsten et al., 1990). Algae cells cannot synthesize DMSP under dark conditions (Stefels, 2000). Marine heterotrophic bacteria are major degraders of DMSP via two pathways: demethylation and cleavage (Zhang et al., 2019). In addition, many phytoplankton and some fungi can also cleave DMSP (Zhang et al., 2019). Therefore, concentrations of DMSP should decrease dramatically with depth below the euphotic layer. However, in this study, average concentrations of DMSPp were 6.6±3.7, 7.1±3.1, 6.3±3.1, and 8.0±3.6 nmol/L in the euphotic layer, the mesopelagic layer, the bathypelagic layer, and the abyssal layer, respectively. The mean concentrations of DMSPt were 8.5±3.4, 8.5±3.2, 7.7±3.1, and 9.3±3.7 nmol/L in the euphotic layer, the mesopelagic layer, the bathypelagic layer, and the abyssal layer, respectively. The results indicate that although phytoplankton could not produce DMSP in the deep ocean, the concentrations of DMSP are still at high levels with relatively consistent values. Similar phenomenon has been found in previous studies. Zheng et al. (2020) reported that concentrations of DMSP ranged 1.0–2.4 nmol/L in the aphotic waters of the Challenger Deep of the Mariana Trench. Li et al. (2015) documented that concentrations of DMSPp varied from 0.1 to 5.7 nmol/L on average of 2.9 nmol/L in 2 600–3 700 m in the Greenland Sea. In addition, concentrations of DMSPp ranged 0.5–22.2 nmol/L on average of 8.6 nmol/L in 3 000 – 4 000 m in the Norwegian Sea (Li et al., 2015). Zhang et al. (2018) suggested that mean concentrations of DMSPp were 1.6±1.8, 1.1±1.0, 1.3±1.4, and 0.3±0.3 nmol/L in the mesopelagic layer, the bathypelagic layer, the abyssal layer, and the hadal zone of the northern Yap Trench, respectively. Their study also showed that average concentrations of DMSPt were 3.1±2.1, 2.5±1.2, 2.4±1.4, and 1.3±0.7 nmol/L in the mesopelagic layer, the bathypelagic layer, the abyssal layer, and the hadal zone of the northern Yap Trench, respectively. A recent research has shown that marine heterotrophic bacteria with dsyB synthesis gene can produce DMSP in the East China Sea (Curson et al., 2017). Zheng et al. (2020) found that bacterial DMSP synthesis via the dsyB gene and transcription of gene was greater in deep seawater and sediment of the Mariana Trench. They proved that heterotrophic bacteria were important DMSP producers in the marine aphotic and high-pressure environments. In addition, DMSP has a physiological function of hydrostatic pressure protection (Zheng et al., 2020). The Yap Trench is adjacent to the Mariana Trench, the channel at the Yap-Mariana Junction is essential for the exchange of deep water (water flowing to East Caroline Basin from East Mariana Basin) (Siedler et al., 2004). The hydrological variations (the isolines of temperature, salinity, and density) in the Yap Trench are similar to those in the Challenger Deep of the Mariana Trench (Liu et al., 2020). The consistency of high concentrations of DMSP below the euphotic layer in the southern Yap Trench is similar to the Mariana Trench (Zheng et al., 2020). Therefore, the consistency of high DMSP in the deep seawater of the southern Yap Trench might be attributed to the role of heterotrophic bacteria production.
4.2 The elevated concentrations of DMSP around 4 000 m affected by the input of LCPWIn the southern Yap Trench, average concentrations of DMSPp and DMSPt were 11.17±0.98 and 12.6±0.8 nmol/L, with the highest value of 12.4 and 13.7 nmol/L around 4 000-m depth, respectively. The average concentrations of DMSPp and DMSPt in 4 000-m depth were conspicuously higher than the average concentrations of 6.7 and 7.8 nmol/L in 3 000-m depth (Fig. 3). The concentrations of total organic carbone (TOC) kept consistent levels between 3 000 and 5 000 m, and had no increasing trend around 4 000-m depth (unpublished data). No evidence is shown that bacterial DMSP producers are more active in 4 000-m depth of the trench. If DMSP completely originates from in-situ production and/or sinking particulate matter of the study area, the sources might not lead to remarkably elevated concentrations of DMSPp and DMSPt in the seawater at 4 000-m depth. Therefore, the input of external DMSP might cause higher concentrations of DMSPp and DMSPt at this depth. Liu et al. (2020) reported that abyssal water in the southern Yap Trench marked by cold, high salinity, and high oxygen concentration was from the Lower Circumpolar Water (LCPW). The high precision profiles of temperature, salinity, DO, and potential density (σ) below 3 000 m at stations C01–C04 are shown in the reference of Liu et al. (2020) (Supplementary Figs.S1–S2). Liu et al. (2018) also found that the deep water in the northern Yap Trench originates from EMB and ECB, as part of the west propagating LCPW. The vertical patterns of temperature, salinity, DO, and potential density in the southern Yap Trench were consistent with the observed results in the northern Yap Trench (Liu et al., 2020). In addition, Kawabe et al. (2003) and Siedler et al. (2004) also confirmed that the current between the Yap Trench and basins of the Mariana-Caroline regions was a part of LCPW below the North Pacific Deep Water (NPDW). High-level concentrations of DMSP in the Antarctic surface water have been documented by several of studies (Table 2). Damm et al. (2016) observed that concentrations of DMSPt ranged from 0.5 to 750.0 nmol/L in the Antarctic Sea Ice. Turner et al. (1995) described that concentrations of DMSPp varied from 2.0 to 69.0 nmol/L in the Bellingshausen Sea, Antarctica. Stefels et al. (2018) proposed that concentrations of DMSPp and DMSPt were 82.0–368.0 nmol/L and 94.0–643.0 nmol/L in the seawater around the West Antarctic Peninsula, respectively. These results indicated that cold water could impoverish DMSP consumption. Then, a part of DMSP might be retained in the sinking-cold Antarctic surface water and transported into the abyssal layer of the Yap Trench with LCPW. Therefore, the elevated concentrations of DMSP around 4 000 m might be affected by the input of LCPW.
In the southern Yap Trench, concentrations of CH4 in the seawater of the Benthic Boundary Layer were slightly higher than those in the water column at approaching depth (Fig. 4), the mean concentration of CH4 in the Benthic Boundary Layer was slightly higher than those values in other layers of the water column, suggesting that the sediment was a weak source of CH4 for the bottom seawater. Fu et al. (2020) reported that sulfate-reducing bacteria (SRB) were detected in the sediment of the southern Yap Trench. The abundance of SRB decreased with increasing depth, and the copy numbers of dsrB (for SRB) genes varied from 0 to 2.33×102 copies/g. Therefore, a part of CH4 produced in the sediment was consumed by SRB, decreasing the release of CH4 to the bottom water. In addition, methanogenesis requires strictly anaerobic and highly reducing environments. The aerobic environment at the bottom of the trench was unfavorable for CH4 production and promoted its oxidation. Then, it is reasonable not to find anomalously high concentrations of CH4 in seawater of the Benthic Boundary Layer.
Concentrations of DMSPp and DMSPt from stations D150, D151, and D152 in the hadal zone were not predominantly higher than those in stations D148 and D149 in the abyssal layer (Fig. 4). Concentrations of DMSPp and DMSPt in the seawater of the Benthic Boundary Layer were not higher than those in the water column at approximate depth. These results indicate that the hadal sediment is not a DMSP source for seawater in the study area. Zheng et al. (2020) reported that DMSP of the sediment from the Challenger Deep of the Mariana Trench were higher than those in the water column at the same depth. Their report is not consistent with our results. The input of LCPW in the abyssal layer of the southern Yap Trench might elevate the concentrations of DMSP in the water column, leading to concentrations of DMSPp and DMSPt in the seawater of the Benthic Boundary Layer were in relatively lower levels when compared with those in the water column.
4.4 CH4 surplus in the surface water—the "Oceanic CH4 Paradox"In the seawater of the southern Yap Trench, concentrations of CH4 ranged 2.0–4.0 nmol/L in the euphotic layer. The range is consistent with 1.8–4.8 nmol/L in the western Pacific Ocean (Zindler et al., 2013), 1.6–3.9 nmol/L in the northern Yap Trench (Zhang et al., 2018), and 1.6–3.6 nmol/L in the Pacific Ocean (Bates et al., 1996). The mean CH4 concentration of 2.8±0.6 nmol/L in the euphotic layer of this study is similar to the concentrations of 2.8±0.6, 2.4±0.5, 2.5, and 2.5 nmol/L in the northern Yap Trench, in the western Pacific Ocean, in the subtropical North Atlantic Ocean, and in the western North Pacific Ocean, respectively (Scranton and Brewer, 1977; Watanabe et al., 1995; Zindler et al., 2013; Zhang et al., 2018). In the water column of the southern Yap Trench, the depth with the highest concentration of CH4 at the subsurface layer (150 m) coincides the thermocline and the halocline. This kind of coincidence is consistent with previous results (Burke et al., 1983; Holmes et al., 2000). In the euphotic layer, CH4 was supersaturated and saturation ranging from 94% to 204%. In general, CH4 surplus is a ubiquitous phenomenon in open ocean (Kelley and Jeffrey, 2002). For example, in the subtropical North Pacific Ocean, the saturation ranged from 105% to 175% in the upper 200 m of the water column (Tilbrook and Karl, 1995); in surface water of the Atlantic Ocean, the value was between 101% and 158% (Conrad and Seiler, 1988); in surface water of the western Pacific Ocean, the saturation ranged from 91% to 218% (Zindler et al., 2013), and in surface water of the southern Pacific Ocean and the Southern Ocean, the values were about 180% (Yoshida et al., 2011).
Despite methanogenesis requires anaerobic and highly reducing environments, CH4 oversaturation is frequently detected in oxic surface seawater in open ocean. This contradictory phenomenon has been termed as "Oceanic CH4 Paradox" (Yoshida et al., 2011). To explain this paradox, previous studies documented that methanogenesis existed in anoxic microenvironment of the guts in zooplankton or fish (de Angelis and Lee, 1994), of sinking particles such as fecal pellets (Tilbrook and Karl, 1995; Holmes et al., 2000; Sasakawa et al., 2008), and inside bacterial cells (Damm et al., 2015). Under these conditions, methanogenesis bacteria could produce CH4 by fermentation of organic molecules. However, Schmale et al. (2018) and Sasakawa et al. (2008)' s results indicated that CH4 production from zooplankton guts was not enough to maintain CH4 surplus in surface water. On the other hand, abundant phytoplankton biomass could produce plenty of particulate organic matter in marine environment. Degradation of these particles could provide conducive conditions for in-situ CH4 production in seawater (Karl and Tilbrook, 1994; Zindler et al., 2013). However, in this study, the relationships between concentrations of CH4 and Chl a were insignificant (C01: R=-0.219; C02: R=-0.038; C03: R=0.204; C04: R=-0.006) (Table 3), indicating that concentration variations of CH4 are not consistent with the amounts of phytoplankton particles in the surface seawater. Zindler et al. (2013) also documented that CH4 surplus was not related to the production of algae in open ocean surface water. Thus, if sinking particles from phytoplankton could provide microenvironment supporting CH4 surplus in oxic surface water of the southern Yap Trench should be remained for further investigation.
|
In recent years, new evidence from series of studies challenged the conventional opinions about the biosynthesis of CH4 in marine environment. For example, Karl et al. (2008) induced that CH4 was produced in aerobic phosphate-stressed seawater as a by-product of methylphosphonate (MPn) decomposition by MPn-amended treatments. Ye et al. (2020) also proved that CH4 was produced from MPn metabolism by MPn-amended incubation in oligotrophic oxic surface water of the western North Pacific Ocean. This process was regulated by the availability of dissolved inorganic phosphate (Pi). Pi-stressed conditions promote microbe to generate CH4 from MPn (Karl et al., 2008; Ye et al., 2020). As well, concentrations of the dissolved Pi were below the detection limit and nutrient levels were very low in the surface water of the southern Yap Trench (unpublished data) (Supplementary Fig. S3). Thus, CH4 production from MPn metabolism might occur in this oxic surface seawater, and the relationships between MPn and CH4 should be retained for further investigation.
Additionally, another potential substrate for CH4 production is DMSP in aerobic environments (Damm et al., 2008; Zindler et al., 2013). In this study, concentrations of CH4 versus DMSPd were conspicuously negatively correlated (C01: R= -0.814; C02: R=-0.848; C03: R=-0.692; C04: R= -0.618), and concentrations of CH4 versus DMSPp were positively related (C01: R=0.622; C03: R= 0.552) in the euphotic layer (Table 3). The positive correlations between CH4 and DMSPp were also reported in the oligotrophic surface seawater of the East China Sea (Zhai et al., 2019). The results might prove that DMSP as a precursor generates CH4 in oxic surface seawater of the southern Yap Trench. Detailed discussions about relationships between CH4 and DMSP in the study area are shown in Section 4.5 below.
4.5 DMSP as a potential precursor for CH4 in the surface seawaterIn the marine environment, DMSP as a potential precursor for CH4 in oxic surface seawater has been proposed by several studies (Damm et al., 2010, 2015; Florez-Leiva et al., 2013). Damm et al. (2008) indicated that DMSP and CH4 are negatively correlated, suggesting that DMSP could be a potential substrate for the methanogenesis in the Storfjorden shelf water—the Arctic shelf region. Damm et al. (2010) reported that CH4 production occurs while DMSP is utilized as a carbon source if nitrate is depleted but phosphate is available as a P source, and verified that DMSP induced CH4 production in the phosphate-replete Arctic seawater. Zindler et al. (2013) also proved that DMSP might serve as a potential substrate for CH4 in the oxic surface layer of the western Pacific Ocean. Damm et al. (2015) documented that CH4 is in situ produced while DMSP released from sea ice may serve as a precursor for the CH4 formation in oxygen-rich polar water. Zhai et al. (2019) reported that significant correlations were found between concentrations of CH4 and DMSPp, and the bacterial communities had potential to utilize DMSPp, meanwhile producing CH4 under oligotrophic conditions in the East China Sea. In the aphotic zone of the study area, positive relationships between concentrations of CH4 and DMSPt (C01: R=0.676; C02: R=0.507; C03: R=0.668; C04: R=0.529), and between concentrations of CH4 and DMSPp (C01: R=0.733; C03: R=0.686) were found (Table 4). Similar positive relationships have been found in the northern Yap Trench below 200 m (Zhang et al., 2018). These positive correlations indicate that DMSP might be a direct precursor of CH4 in aphotic layer of the southern Yap Trench, and further support previous conclusions about DMSP as precursor of CH4 in oxic seawater. In the study area, DMSPp was the major fraction of DMSPt. Particulate matter might provide a conducive microenvironment for CH4 generation from DMSP. Therefore, it is reasonable to find positive relationships between concentrations of CH4 and DMSPp, and between concentrations of CH4 and DMSPt in the seawater. In order to prove the possibility of DMSP as substrate for CH4 production, it is necessary to carry out isotopic tracer incubation experiment in the future.
|
In the southern Yap Trench, the difference of correlations between concentrations of CH4 and DMSPd, and between concentrations of CH4 and DMSPp reflects the complex transformation among CH4, DMSPd, and DMSPp in the euphotic layer. Significant negative correlations (C01: R=-0.814; C02: R=-0.848; C03: R=-0.692; C04: R=-0.618) between concentrations of CH4 and DMSPd only occurred in the euphotic layer (Table 3). This kind of negative relationship in the surface seawater is consistent with the results in the northern Yap Trench (Zhang et al., 2018). DMSPd, as a precursor, might be consumed by generating CH4. A possible source of DMSPd is released from DMSPp via algal senescence, viral lysis, and grazing (Christaki et al., 1996; Kiene et al., 2000). DMSPp could deposit or migrate with particles in seawater, which limits the supply of DMSPd from DMSPp. Considering the gradually decreased trend of DMSPd with depth in this layer, negative relationships might be attributed to the consumption of DMSPd by generating CH4 and the limited supply of DMSPd from DMSPp. However, when concentrations of DMSPd were lower than the possible threshold, CH4 production rates from DMSPd decreased dramatically and the strong negative relationships disappeared. Damm et al. (2008) reported an inversely correlation between concentrations of CH4 and DMSPt in the Arctic Ocean surface water when concentrations of DMSPt were higher than the threshold, and the relationship disappeared when the concentrations were lower than the threshold. The remarkable negative relationships between concentrations of CH4 and DMSPd (C01: R=-0.814; C02: R=-0.848; C03: R= -0.692; C04: R=-0.618) in the euphotic layer of the study area indicate that the concentrations of DMSPd were over the threshold for producing CH4. On the other hand, both DMSPd and CH4 in seawater might originate from DMSPp, suggesting that DMSPd might be a CH4 producer and/or a competitor in surface water of the southern Yap Trench. If DMSPd is a CH4 competitor, it is reasonable that concentrations of CH4 versus DMSPd are negatively correlated.
In general, the possible cycling of CH4 and DMSP from surface seawater to bottom seawater of the southern Yap Trench is present in Fig. 5.
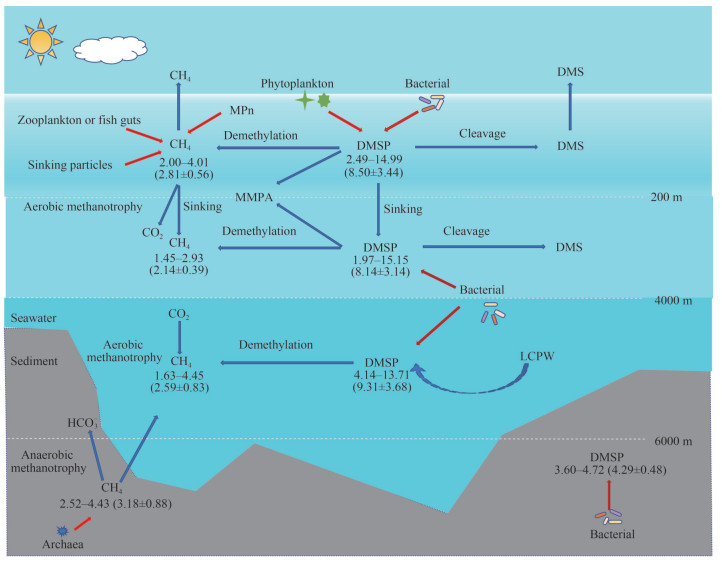
|
| Fig.5 The possible cycling of CH4 and DMSP from the surface seawater to the bottom seawater of the southern Yap Trench Blue arrows represent removal pathway and red arrows represent production pathway. Values in this figure represent the concentration of CH4 and DMSP ranges in this study, unit: nmol/L. Values in parentheses represent the average and standard deviation, unit: nmol/L. |
Based on the vertical profiles of CH4 and DMSP in the southern Yap Trench, CH4 was surplus with respect to the atmosphere in the euphotic layer. Concentrations of DMSPd in the surface layer were higher than those in other layers, and decreased with depth in the euphotic layer of the trench. Concentrations of DMSPp and DMSPt shared similar increasing trend in the euphotic layer and decreased inconspicuously in 200–3 000-m depth, which might attribute to the production of heterotrophic bacteria in aphotic and high-pressure deep water. An exception occurred around 4 000 m where their concentrations increased predominately and decreased rapidly below 4 000-m depth, suggesting that elevated concentrations of DMSP in the abyssal layer might be affected by the LCPW. The sediment was a weak source of CH4 and was not a source of DMSP for the water column of the trench. Predominately negative relationships between CH4 and DMSPd were presented in the surface water. Concentrations of CH4 versus DMSPp were positively related in both the euphotic layer and the aphotic layer below 200-m depth. Positive relationships between concentrations of CH4 and DMSPt were also found below 200-m depth. The results suggested that DMSP might be a potential substrate of CH4 not only in the oxic surface water but also in the dark deep water. The results of this study will expand understanding of the biogeochemical cycle of CH4 and DMSP from the sea surface to the hadal zone of the southern Yap Trench.
6 DATA AVAILABILITY STATEMENTThe data are available online (https://doi.org/10.6084/m9.figshare.16699066).
7 ACKNOWLEDGMENTWe are grateful to Wei CAO from Marine Bioresource and Environment Research Center, the First Institute of Oceanography, Ministry of Natural Resource for sample collecting and to Haorui LIANG from South China Environment Monitoring Center, State Oceanic Administration for constructive comments.
Electronic supplementary material
Supplementary material (Supplementary Table S1 and Figs.S1–S3) is available in the online version of this article at https://doi.org/10.1007/s00343-022-2063-8.
Bates T S, Kelly K C, Johnson J E, et al. 1996. A reevaluation of the open ocean source of methane to the atmosphere. Journal of Geophysical Research: Atmospheres, 101(D3): 6953-6961.
DOI:10.1029/95JD03348 |
Beccaluva L, Macciotta G, Savelli C et al. 1980. Geochemistry and K/Ar ages of volcanics dredged in the Philippine Sea (Mariana, Yap, and Palau trenches and Parece Vela basin). In: Hayes D E ed. The Tectonic and Geologic Evolution of Southeast Asian Seas and Islands, Volume 23. American Geophysical Union, Washington, D.C. p. 247-268, https://doi.org/10.1029/GM023p0247.
|
Burke R A Jr, Reid D F, Brooks J M, et al. 1983. Upper water column methane geochemistry in the eastern tropical North Pacific. Limnology and Oceanography, 28(1): 19-32.
DOI:10.4319/lo.1983.28.1.0019 |
Christaki U, Belviso S, Dolan J R, et al. 1996. Assessment of the role of copepods and ciliates in the release to solution of particulate DMSP. Marine Ecology Progress Series, 141: 119-127.
DOI:10.3354/meps141119 |
Conrad R, Seiler W. 1988. Methane and hydrogen in seawater (Atlantic Ocean). Deep Sea Research Part A. Oceanographic Research Papers, 35(12): 1903-1917.
DOI:10.1016/0198-0149(88)90116-1 |
Cui Y S, Suzuki S, Omori Y, et al. 2015. Abundance and distribution of dimethylsulfoniopropionate degradation genes and the corresponding bacterial community structure at dimethyl sulfide hot spots in the tropical and subtropical Pacific Ocean. Applied and Environmental Microbiology, 81(12): 4184-4194.
DOI:10.1128/AEM.03873-14 |
Curson A R J, Liu J, Bermejo Martínez A, et al. 2017. Dimethylsulfoniopropionate biosynthesis in marine bacteria and identification of the key gene in this process. Nature Microbiology, 2(5): 17009.
DOI:10.1038/nmicrobiol.2017.9 |
Dacey J W H, Blough N V. 1987. Hydroxide decomposition of dimethylsulfoniopropionate to form dimethylsulfide. Geophysical Research Letters, 14(12): 1246-1249.
DOI:10.1029/GL014i012p01246 |
Damm E, Helmke E, Thoms S, et al. 2010. Methane production in aerobic oligotrophic surface water in the central Arctic Ocean. Biogeosciences, 7(3): 1099-1108.
DOI:10.5194/bg-7-1099-2010 |
Damm E, Kiene R P, Schwarz J, et al. 2008. Methane cycling in Arctic shelf water and its relationship with phytoplankton biomass and DMSP. Marine Chemistry, 109(1-2): 45-59.
DOI:10.1016/j.marchem.2007.12.003 |
Damm E, Nomura D, Martin A, et al. 2016. DMSP and DMS cycling within Antarctic sea ice during the winter-spring transition. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 131: 150-159.
DOI:10.1016/j.dsr2.2015.12.015 |
Damm E, Thoms S, Beszczynska-Möller A, et al. 2015. Methane excess production in oxygen-rich polar water and a model of cellular conditions for this paradox. Polar Science, 9(3): 327-334.
DOI:10.1016/j.polar.2015.05.001 |
de Angelis M A, Lee C. 1994. Methane production during zooplankton grazing on marine phytoplankton. Limnology and Oceanography, 39(6): 1298-1308.
DOI:10.4319/lo.1994.39.6.1298 |
Ding H B, Sun C J. 2020. Towards the understanding from sea surface to hadal zone—a multidisciplinary study of the Yap Trench. Journal of Oceanology and Limnology, 38(3): 591-592.
DOI:10.1007/s00343-020-0591-7 |
Florez-Leiva L, Damm E, Farías L. 2013. Methane production induced by dimethylsulfide in surface water of an upwelling ecosystem. Progress in Oceanography, 112-113: 38-48.
DOI:10.1016/j.pocean.2013.03.005 |
Fu L L, Li D, Mi T Z, et al. 2020. Characteristics of the archaeal and bacterial communities in core sediments from Southern Yap Trench via in situ sampling by the manned submersible Jiaolong. Science of the Total Environment, 703: 134884.
DOI:10.1016/j.scitotenv.2019.134884 |
Fujiwara T, Tamura C, Nishizawa A, et al. 2000. Morphology and tectonics of the Yap Trench. Marine Geophysical Researches, 21(1-2): 69-86.
DOI:10.1023/a:1004781927661 |
Holmes M E, Sansone F J, Rust T M, et al. 2000. Methane production, consumption, and air-sea exchange in the open ocean: an evaluation based on carbon isotopic ratios. Global Biogeochemical Cycles, 14(1): 1-10.
DOI:10.1029/1999GB001209 |
IP CC. 2013. Climate change 2013: the physical science basis. Cambridge University Press, New York. p. 159-218.
|
Jayakumar D A, Naqvi S W A, Narvekar P V, et al. 2001. Methane in coastal and offshore waters of the Arabian Sea. Marine Chemistry, 74(1): 1-13.
DOI:10.1016/S0304-4203(00)00089-X |
Johnson G C, Toole J M. 1993. Flow of deep and bottom waters in the Pacific at 10°N. Deep Sea Research Part Ⅰ: Oceanographic Research Papers, 40(2): 371-394.
DOI:10.1016/0967-0637(93)90009-R |
Kaneko I, Takatsuki Y, Kamiya H, et al. 1998. Water property and current distributions along the WHP-P9 section (137°–142°) in the western North Pacific. Journal of Geophysical Research: Oceans, 103(C6): 12959-12984.
DOI:10.1029/97JC03761 |
Karl D M, Beversdorf L, Björkman K M, et al. 2008. Aerobic production of methane in the sea. Nature Geoscience, 1(7): 473-478.
DOI:10.1038/ngeo234 |
Karl D M, Tilbrook B D. 1994. Production and transport of methane in oceanic particulate organic matter. Nature, 368(6473): 732-734.
DOI:10.1038/368732a0 |
Karsten U, Wiencke C, Kirst G O. 1990. The β-dimethylsulphoniopropionate (DMSP) content of Macroalgae from Antarctica and Southern Chile. Botanica Marina, 33(2): 143-146.
DOI:10.1515/botm.1990.33.2.143 |
Kawabe M, Fujio S, Yanagimoto D. 2003. Deep-water circulation at low latitudes in the western North Pacific. Deep Sea Research Part Ⅰ: Oceanographic Research Papers, 50(5): 631-656.
DOI:10.1016/S0967-0637(03)00040-2 |
Kawabe M, Taira K. 1998. Water masses and properties at 165°E in the western Pacific. Journal of Geophysical Research: Oceans, 103(C6): 12941-12958.
DOI:10.1029/97JC03197 |
Kelley C A, Jeffrey W H. 2002. Dissolved methane concentration profiles and air-sea fluxes from 41° S to 27°N. Global Biogeochemical Cycles, 16(3): 13-1.
DOI:10.1029/2001GB001809 |
Kettle A J, Andreae M O. 2000. Flux of dimethylsulfide from the oceans: a comparison of updated data sets and flux models. Journal of Geophysical Research: Atmospheres, 105(D22): 26793-26808.
DOI:10.1029/2000JD900252 |
Kiene R P, Linn L J, Bruton J A. 2000. New and important roles for DMSP in marine microbial communities. Journal of Sea Research, 43(3-4): 209-224.
DOI:10.1016/S1385-1101(00)00023-X |
Kiene R P, Slezak D. 2006. Low dissolved DMSP concentrations in seawater revealed by small-volume gravity filtration and dialysis sampling. Limnology and Oceanography: Methods, 4(4): 80-95.
DOI:10.4319/lom.2006.4.80 |
Lee S M. 2004. Deformation from the convergence of oceanic lithosphere into Yap trench and its implications for early-stage subduction. Journal of Geodynamics, 37(1): 83-102.
DOI:10.1016/j.jog.2003.10.003 |
Lenhart K, Klintzsch T, Langer G, et al. 2016. Evidence for methane production by the marine algae Emiliania huxleyi. Biogeosciences, 13(10): 3163-3174.
DOI:10.5194/bg-13-3163-2016 |
Li C X, Wang B D, Yang G P. 2015. Spatial distributions of dimethylsulfide (DMS) and influencing factors in the Norwegian and Greenland Seas during summer. Haiyang Xuebao, 37(8): 9-25.
(in Chinese with English abstract) DOI:10.3969/j.issn.0253-4193.2015.08.002 |
Li Y H, Zhan L Y, Zhang J X, et al. 2017. A significant methane source over the Chukchi Sea shelf and its sources. Continental Shelf Research, 148: 150-158.
DOI:10.1016/j.csr.2017.08.019 |
Liu X H, Liu Y Z, Cao W, et al. 2020. Water characteristics of abyssal and hadal zones in the southern Yap Trench observed with the submersible Jiaolong. Journal of Oceanology and Limnology, 38(3): 593-605.
DOI:10.1007/s00343-019-8368-6 |
Liu Y Z, Liu X H, Lv X Q, et al. 2018. Watermass properties and deep currents in the northern Yap Trench observed by the Submersible Jiaolong system. Deep Sea Research Part Ⅰ: Oceanographic Research Papers, 139: 27-42.
DOI:10.1016/j.dsr.2018.06.001 |
Reeburgh W S. 2007. Oceanic methane biogeochemistry. Chemical Reviews, 107(2): 486-513.
DOI:10.1021/cr050362v |
Rellinger A N, Kiene R P, del Valle D A, et al. 2009. Occurrence and turnover of DMSP and DMS in deep waters of the Ross Sea, Antarctica. Deep Sea Research Part Ⅰ: Oceanographic Research Papers, 56(5): 686-702.
DOI:10.1016/j.dsr.2008.12.010 |
Sasakawa M, Tsunogai U, Kameyama S, et al. 2008. Carbon isotopic characterization for the origin of excess methane in subsurface seawater. Journal of Geophysical Research: Oceans, 113(C3): C03012.
DOI:10.1029/2007JC004217 |
Schmale O, Wäge J, Mohrholz V, et al. 2018. The contribution of zooplankton to methane supersaturation in the oxygenated upper waters of the central Baltic Sea. Limnology and Oceanography, 63(1): 412-430.
DOI:10.1002/lno.10640 |
Scranton M I, Brewer P G. 1977. Occurrence of methane in the near-surface waters of the western subtropical North-Atlantic. Deep Sea Research, 24(2): 127-138.
DOI:10.1016/0146-6291(77)90548-3 |
Siedler G, Holfort J, Zenk W, et al. 2004. Deep-water flow in the Mariana and Caroline Basins. Journal of Physical Oceanography, 34(3): 566-581.
DOI:10.1175/2511.1 |
Stefels J. 2000. Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. Journal of Sea Research, 43(3-4): 183-197.
DOI:10.1016/S1385-1101(00)00030-7 |
Stefels J, van Leeuwe M A, Jones E M, et al. 2018. Impact of sea-ice melt on dimethyl sulfide (sulfoniopropionate) inventories in surface waters of Marguerite Bay, West Antarctic Peninsula. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 376(2122): 20170169.
DOI:10.1098/rsta.2017.0169 |
Tilbrook B D, Karl D M. 1995. Methane sources, distributions and sinks from California coastal waters to the oligotrophic North Pacific gyre. Marine Chemistry, 49(1): 51-64.
DOI:10.1016/0304-4203(94)00058-L |
Turner S M, Nightingale P D, Broadgate W, et al. 1995. The distribution of dimethyl sulphide and dimethylsulphoniopropionate in Antarctic waters and sea ice. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 42(4-5): 1059-1080.
DOI:10.1016/0967-0645(95)00066-Y |
Valentine D L. 2011. Emerging topics in marine methane biogeochemistry. Annual Review of Marine Science, 3(1): 147-171.
DOI:10.1146/annurev-marine-120709-142734 |
Watanabe S, Higashitani N, Tsurushima N, et al. 1995. Methane in the western North Pacific. Journal of Oceanography, 51(1): 39-60.
DOI:10.1007/BF02235935 |
Wiesenburg D A, Guinasso N L. 1979. Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water. Journal of Chemical & Engineering Data, 24(4): 356-360.
DOI:10.1021/je60083a006 |
Yang G P, Zhang H H, Zhou L M, et al. 2011. Temporal and spatial variations of dimethylsulfide (DMS) and dimethylsulfoniopropionate (DMSP) in the East China Sea and the Yellow Sea. Continental Shelf Research, 31(13): 1325-1335.
DOI:10.1016/j.csr.2011.05.001 |
Ye W W, Wang X L, Zhang X H, et al. 2020. Methane production in oxic seawater of the western North Pacific and its marginal seas. Limnology and Oceanography, 65(10): 2352-2365.
DOI:10.1002/lno.11457 |
Yoch D C. 2002. Dimethylsulfoniopropionate: its sources, role in the marine food web, and biological degradation to dimethylsulfide. Applied and Environmental Microbiology, 68(12): 5804-5815.
DOI:10.1128/AEM.68.12.5804-5815.2002 |
Yoshida O, Inoue H Y, Watanabe S, et al. 2011. Dissolved methane distribution in the South Pacific and the Southern Ocean in austral summer. Journal of Geophysical Research: Oceans, 116(C7): C07008.
DOI:10.1029/2009JC006089 |
Zhai X, Li J L, Zhang H H, et al. 2019. Spatial distribution and biogeochemical cycling of dimethylated sulfur compounds and methane in the East China Sea during spring. Journal of Geophysical Research: Oceans, 124(2): 1074-1090.
DOI:10.1029/2018JC014488 |
Zhai X, Zhang H H, Yang G P, et al. 2018. Distribution and sea-air fluxes of biogenic gases and relationships with phytoplankton and nutrients in the central basin of the South China Sea during summer. Marine Chemistry, 200: 33-44.
DOI:10.1016/j.marchem.2018.01.009 |
Zhang G L, Zhang J, Liu S M, et al. 2008. Methane in the Changjiang (Yangtze River) Estuary and its adjacent marine area: riverine input, sediment release and atmospheric fluxes. Biogeochemistry, 91(1): 71-84.
DOI:10.1007/s10533-008-9259-7 |
Zhang M J, Sun C J, Yang G P, et al. 2018. The vertical variation characteristics of CH4 and DMSP in the seawater of the Yap Trench in the western Pacific Ocean. Haiyang Xuebao, 40(10): 143-157.
(in Chinese with English abstract) DOI:10.3969/ji.ssn.0253-4193.2018.10.014 |
Zhang X H, Liu J, Liu J L, et al. 2019. Biogenic production of DMSP and its degradation to DMS—their roles in the global sulfur cycle. Science China Life Sciences, 62(10): 1296-1319.
DOI:10.1007/s11427-018-9524-y |
Zheng Y F, Wang J Y, Zhou S, et al. 2020. Bacteria are important dimethylsulfoniopropionate producers in marine aphotic and high-pressure environments. Nature Communications, 11(1): 4658.
DOI:10.1038/s41467-020-18434-4 |
Zindler C, Bracher A, Marandino C A, et al. 2013. Sulphur compounds, methane, and phytoplankton: interactions along a north-south transit in the western Pacific Ocean. Biogeosciences, 10(5): 3297-3311.
DOI:10.5194/bg-10-3297-2013 |
 2023, Vol. 41
2023, Vol. 41