Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CUI Dong, SUN Chengjun, GUO Chaonan, CAO Wei, JIANG Fenghua, DING Haibing
- Characteristics of dissolved sugars in the Southern Yap Trench from sea surface to hadal zone
- Journal of Oceanology and Limnology, 41(6): 2117-2133
- http://dx.doi.org/10.1007/s00343-023-2176-8
Article History
- Received Apr. 7, 2022
- accepted in principle May 24, 2022
- accepted for publication Nov. 21, 2022
2 Marine Ecology and Environmental Science Laboratory, Laoshan Laboratory, Qingdao 266237, China;
3 First Institute of Oceanography, Ministry of Natural Resources, Qingdao 266061, China
Sugars (carbohydrate) are important and relatively unstable components of marine organic matter (Hung et al., 2001; Arnosti et al., 2021), playing key roles in the metabolism of plankton (Zhang and Fang, 1991), and playing critical roles in maintaining marine food chain. In marine environment, sugars can be converted into other essential components in organisms, such as proteins, lipids, and nucleic acids (Myklestad and Børsheim, 2007), and can also provide energy for various life activities through biochemical processes such as respiration (Witter and Luther III, 2002). Sugars in seawater are derived from phytoplankton photosynthesis (Kerhervé et al., 2002; Thornton, 2014), plankton excretion and death decomposition (Burney et al., 1979), bacterial release (Kawasaki and Benner, 2006), sediment resuspension, and etc. (Arnosti and Holmer, 1999). Exploring the distributions, sources, and migrations of sugars in marine environment is of great significance for the study of marine organic carbon cycle.
Dissolved organic carbon (DOC) in seawater is an important indicator for the study of biogeochemistry (Hung et al., 2000; Ji et al., 2019), playing vital roles in the ocean carbon cycle, and also has non-negligible significance on global carbon cycle (Carlson et al., 1994). DOC in the ocean includes labile DOC and recalcitrant DOC. Dissolved carbohydrates are an important part of marine labile DOC, including dissolved monosaccharides (MCHO) and polysaccharides (PCHO). At present, studies on marine soluble sugars have been carried out in many sea areas, including the Northern Yap Trench (Guo et al., 2020), the equatorial Pacific Ocean (Pakulski and Benner, 1994), the Arctic Ocean (Wang et al., 2006), the coast of the Western Antarctic Peninsula (Zeppenfeld et al., 2021), the Indian Ocean (Khodse et al., 2007), the Gulf of Mexico (Hung et al., 2003), a range of different types of estuaries (Hung et al., 2005), and other coastal regions (Witter and Luther III, 2002; Myklestad and Børsheim, 2007; He et al., 2015; Ji et al., 2019). Previous study also investigated the control factors on the distribution of sugars in marine environments, including photosynthesis, bacterial degradation, terrestrial input, sediment resuspension, aerosol deposition, and etc. (He et al., 2015; Arnosti et al., 2021; Zeppenfeld et al., 2021). However, the study of carbohydrate in marine environment is still very limited, especially in bathypelagic region, abyss, and hadal zone. Guo et al. (2020) studied the sugars for the first time in the Yap Trench, and systematically described the fundamental characteristics of the distributions and the possible sources of sugars in its northern region. But so far, little study was conducted on the characteristics of sugars from the euphotic layer to the hadal zone in marine extreme environment.
The Yap Trench is one of the deepest trenches in the world. The north and the south regions of the Yap Trench is significantly different because three plates (the Pacific Plate, the Philippine Plate, and the Caroline Plate) converge in its north region and two plates (the Philippine Plate and the Caroline Plate) converge in its south region. Over the past few decades, a series of studies have been carried out on its rock composition, water environment characteristics, geological structure and evolution (Hawkins and Batiza, 1977; Johnson and Toole, 1993; Sato et al., 1997; Fujiwara et al., 2000; Guo et al., 2018), but studies on its biogeochemical characteristics are lacking. In recent years, important progress has been made in the study of the biogeochemical characteristics of the Northern Yap Trench. For example, Yan et al. (2020) explored distribution characteristics of lipids in hadal sediment in the Yap Trench. Huang et al. (2020) explored geochemical characteristics of hadal sediment in the Northern Yap Trench. Zhai et al. (2022) studied the microbial community of the trench by investigating the biofilm formed in different metallic surfaces. The water characteristics of the abyss and hadal zone of the trench were analyzed by Liu et al.(2020, 2022). They considered that the upper and lower Circumpolar Deep Waters (UCPW, LCPW) was identified as the main abyssal water masses in the trench and its adjacent region and the hadal water seemed to be of the isolated local water in the Southern Yap Trench. Although Guo et al. (2020) has studied the source and distribution characteristics of carbohydrates in the northern trench, it is necessary to study the features of sugars in its southern region due to the obvious difference of the two regions. This paper is a preliminary study on the biogeochemical characteristics of dissolved sugars in the Southern Yap Trench from the sea surface to the hadal seawater, including their concentrations in seawater, their vertical variation profiles with depth, their sources and sinks, and the controlling factors affecting their distribution characteristics. On the basis of this study, the overall situation of the distributions of dissolved sugars in the Yap Trench was fully grasped through the comparative study with the distribution characteristics of sugars in the Northern Yap Trench by Guo et al. (2020). This study is significant for further understanding the biogeochemical characteristics of labile organic carbon in marine extreme environments, and also provides fundamental data for understanding deep-sea organic carbon cycle in the future.
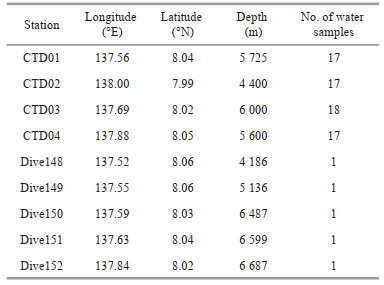
2 MATERIAL AND METHOD 2.1 Study areaThe Yap Trench is located at the junction of the Philippine, the Pacific and the Caroline Plates and is shaped like a letter "J". The trench is 650 km long from north to south, and its deepest point is 8 527 m. Its northern part is connected to the Mariana Trench, which is formed by the subduction of the Pacific Plate and the Caroline Plate under the Philippine Plate (Altis, 1999); and its southern part is connected to the Palau Trench, which is formed by the subduction of the Caroline Plate under the Philippine Plate (Seno et al., 1993). The Yap Trench is affected by different subducting plates and has formed special plate tectonic features, so its abyssal layer and hadal zone have become the frontier for studying marine extreme environment. The Yap Trench has typical deep sea (water depth 1 000–4 000 m), abyss (water depth 4 000–6 000 m), and hadal environment (water depth > 6 000 m). The seawater samples were obtained at nine stations in the study area (Fig. 1), including 4 CTD stations and 5 Dive stations. The fundamental data of the maps in Fig. 1 came from the sharing of the National Deep Sea Center, Ministry of Natural Resources of China. The basic information of the 4 CTD stations and 5 deep diving stations is shown in Table 1.

|
| Fig.1 Locations of the sampling stations in the Southern Yap Trench a. the geographical location of the Yap Trench; b. locations of the sampling stations in the Southern Yap Trench. PAC: Pacific Plate; CP: Caroline Plate; MT: Mariana Trench; YT: Yap Trench; PT: Palau Plate; CR: Caroline Ridge; YI: Yap Island. |
From June 3 to June 13, 2017, R /V Xiangyanghong 09 carrying Jiaolong submersible conducted the 38th survey of the China Ocean Minerals Association in the Southern Yap Trench. The Seabird CTD 911 plus sampler (which can obtain seawater sample from 6 800-m depth directly) carried by R/V Xiangyanghong 09 collected water samples from different water layers at the 4 CTD stations, and the manned Jiaolong submersible which carried an airtight seawater sampler, collected water samples from the sediment-seawater interface at the 5 Dive stations. To avoid disturb of resuspended sediment at the sediment-seawater interface, after the submersible sat on the bottom, the diver detected the surrounding environment until the resuspended sediment disappeared. Then the robotic arm carried by the submersible obtained overlying seawater samples with the sampler outside the influence range of the resuspended sediment. After the samples were collected in glass bottles, 300 mL of the seawater was filtered through a Whatman GF/F filter (47 mm in diameter, 0.7 μm in pore size, glass fiber membrane) on board. Filtered seawater was stored in acid-washed glass bottles and immediately frozen in a -20-℃ freezer for analysis of dissolved sugars. All the glass bottles for seawater sample storage and treatment and the glass fiber were successively washed by different solvents (methanol, dichloromethane, and hexane), and then combusted at 450 ℃ for 5 h.
2.3 Carbohydrates analysisThe concentration of MCHO in the seawater was determined using the 2, 4, 6-tripyridine-s-triazine (TPTZ) method (Myklestad et al., 1997). Briefly, 1 mL of the filtered seawater sample was mixed with 1 mL of 0.7-mmol/L potassium ferricyanide solution in an acid-washed test tube, and placed in a 100-℃ water bath for 10 min. The potassium ferricyanide was prepared in 400-mg NaOH, 20-g Na2CO3, and 230-mg K3[Fe (CN)6] per liter of solution, using organic-free Milli-Q water as solvent. Then 1 mL of 2.0-mmol/L ferric chloride solution and 2 mL of 2.5-mmol/L TPTZ solution were added immediately and mixed well on a vortex mixer. The ferric chloride was prepared in 164-g sodium acetate (anhydrous), 42-g citric acid, and 300-g acetic acid per liter of solution, using organic-free Mili-Q water as solvent, and the TPTZ was prepared in 3-mol/L acetic acid solution. The TPTZ should be prepared within one week before use. The absorbance was measured at 596 nm with an ultraviolet spectrophotometer (UV-2550, Shimadzu Co., Japan). For drawing the working curve, 1-mL D-glucose standard solution with concentrations of 0.00, 2.22, 4.44, 6.66, 8.88, and 11.1 μmol/L, were mixed with 1 mL of the potassium ferricyanide solution respectively. After that, the sample color development procedure and absorbance measurement steps were the same as those of MCHO, the concentrations of MCHO were determined according to the working curve of the D-glucose solution. This method was also used to determine the concentration of TCHO after acid hydrolysis of seawater (4 mL of seawater was measured out, then 0.4 mL 1-mol/L HCl was added in a sealed ampoule, and heated at 150 ℃ for 1 h). The PCHO concentration was the difference between the TCHO and MCHO concentrations ([PCHO]=[TCHO]–[MCHO]). To obtain the best precision, the analytical procedure must be conducted in the dark after adding the potassium ferricyanide solution. The coefficient of variation of the method was 2%–10%, and the detection limit was 2.2 μmol/L using 1-cm cuvette. Glucose equivalents (mmol/L) were converted into carbon (mmol C/L) by multiplying a factor of 6 (according to the molecular structure of glucose) assuming that all monosaccharides in the seawater were hexoses (Myklestad et al., 1997; Bhosle et al., 1998; Görs et al., 2007). For avoiding pollution of organic matter, all the used glassware without scale were washed successively by methanol, dichloromethane, and hexane, and then combusted at 450 ℃ for 5 h.
2.4 Obtaining other parametersIn the cruise, temperature and salinity of the seawater were measured in situ by the ship-based CTD sampler, and dissolved oxygen (DO) concentrations were determined using carried dissolved oxygen sensor. The accuracy of the CTD is 0.001 ℃ for temperature and 0.000 3 S/m for conductivity. The accuracy of the DO sensor is 0.1 mg/L. The observation data, which were managed by the National Deep Sea Center, Ministry of Natural Resource of China, were reprocessed with Sea-Bird equipment (SBE) Data Processing Software V7.2 to obtain the values of temperature, salinity, and DO concentrations. The chlorophyll-a (Chl-a) concentration analysis was conducted by the Second Institute of Oceanography, Ministry of Natural Resource of China using extraction-fluorescence method based on the National Standards of the People's Republic of China (GB12763.6-2007, Specification for Oceanographic Survey Part 6: Marine Biological Survey). In this study, the acquisition and application of the data of temperature, salinity, and concentrations of DO and Chl a were approved by the owners.
Statistical analyses (Pearson's correlation and significance testing) were performed using the SPSS20 software. The sampling map was constructed using the Ocean Data View software (Schlitzer, 2018), and other figures were drawn using Origin 2017 software.
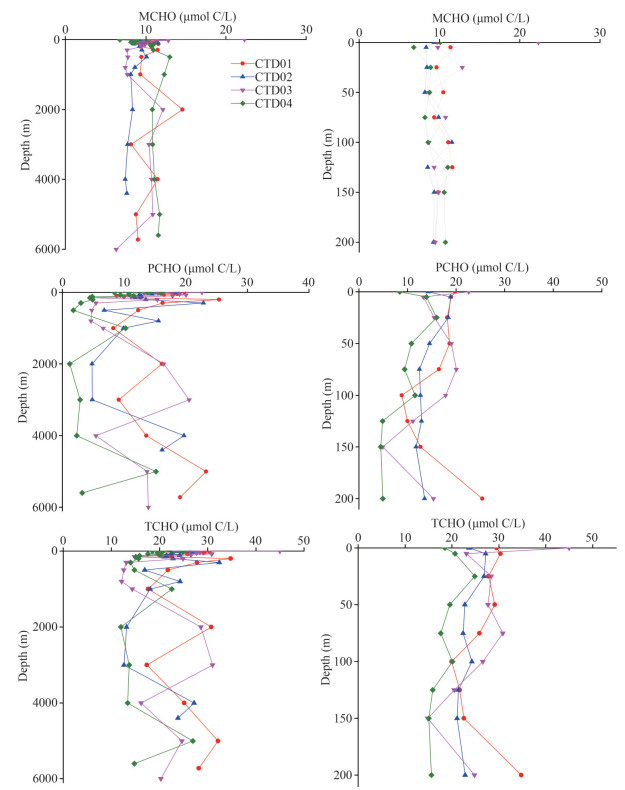
3 RESULT 3.1 Vertical distribution characteristics of dissolved carbohydrates in the Southern Yap TrenchThe concentrations of MCHO, PCHO, and TCHO in the seawater samples from the four CTD stations in the study area ranged from 6.3 to 22.3, 1.1 to 25.4, and 12.0 to 44.9 μmol C/L, respectively. The depths of the maximum concentrations of MCHO, PCHO, and TCHO appeared in the surface seawater at station CTD03 (0 m, 22.3 μmol C/L), the seawater at 200-m depth in station CTD01 (27.3 μmol C/L), and the surface seawater at station CTD03 (0 m, 44.9 μmol C/L), respectively. In addition, their lowest concentrations appeared in station CTD03 at 6 000-m water layer (6.3 μmol C/L), station CTD04 at 2 000-m water layer (1.1 μmol C/L), and station CTD04 at 2 000-m water layer (12.0 μmol C/L), respectively. The vertical variation profiles of MCHO, PCHO, and TCHO concentrations from the surface layer to the hadal zone at the four CTD stations in the study area are shown in Fig. 2.
|
| Fig.2 Vertical variations of the concentrations of MCHO, PCHO, and TCHO in the seawater from the sea surface to the hadal zone and the 0–200-m euphotic layer of the four CTD stations of the Southern Yap Trench |
In the Southern Yap Trench, the variation profiles of the MCHO concentrations showed complex variation trends with depth. Their overall changes in the euphotic layer from 0 to 200 m was insignificant, and the variation trends at the four CTD stations were similar. In general, the MCHO concentrations in the surface seawater of the Southern Yap Trench (5.9–20.9 μmol C/L) were similar to those of the Northern Yap Trench (3.5–27.9 μmol C/L, Guo et al., 2020) and the Indian Ocean (2.4–15.6 μmol C/L, Bhosle et al., 1998), but higher than those in the seawater of the equatorial Pacific Ocean (0.8–7.8 μmol C/L, Pakulski and Benner, 1994), the Atlantic Ocean (4.0–11.0 μmol C/L, Hung et al., 2003) and the Arctic Ocean (0.4–8.6 μmol C/L, Wang et al., 2006). In the mesopelagic layer of 200–1 000 m, the MCHO concentrations remained stable in stations CTD01 and CTD03. At the depth above 1 000 m, the vertical variation trends of MCHO concentrations in stations CTD01 and CTD03 were similar. The concentrations increased firstly and then decreased with water depth, and reached a maximum value at 2 000 m. At stations CTD02 and CTD04, the MCHO concentrations were rarely changed and maintained at low level in the seawater below 2 000-m depth.
The variation trends of the PCHO concentrations in the euphotic layer of the study area was relatively complex. The trends were similar at stations CTD01 and CTD03, decreasing firstly and then increasing with water depth. At station CTD02, the PCHO concentrations changed insignificantly with water depth and maintained at about 13.0 μmol C/L in general. In the 200–1 000-m mesopelagic layer, the concentration of PCHO decreased significantly with water depth at first, and then increased at all the four stations. In stations CTD01 and CTD03, its concentrations reached the second highest value at 2 000–3 000 m. In stations CTD02 and CTD04, the PCHO concentrations had a maximum value in the 4 000–5 000-m water layer, and then decreased with water depth below it. The overall PCHO concentrations in the seawater of the Southern Yap Trench (1.2–25.4 μmol C/L, this study) were close to those in the seawater of the Northern Yap Trench (3.5–27.9 μmol C/L, Guo et al., 2020), the Pacific Ocean (2.6–25.8 μmol C/L, Pakulski and Benner, 1994), and the upper Atlantic Ocean (0–500 m) (2.0–16.0 μmol C/L, Hung et al., 2003), but higher than those in the surface seawater of the Arctic Ocean (0.5–13.6 μmol C/L, Wang et al., 2006).
From 0- to 6 000-m water depth, the vertical profiles of the TCHO concentrations showed fluctuating trends in the four stations. The trends were similar at stations CTD01 and CTD03 with one pattern, and were similar at stations CTD02 and CTD04 with another pattern. On the whole, the trends of the TCHO concentrations with water depth were consistent with those of the PCHO concentrations at the same station.
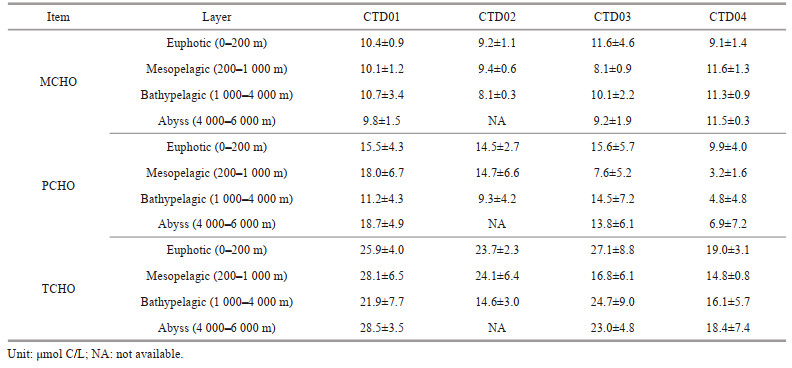
3.2 Average concentration of sugars in different water layers of the four CTD stationsThe average concentrations of MCHO, PCHO, and TCHO in the euphotic, mesopelagic, bathypelagic, and abyssal layers of the four CTD stations in the Southern Yap Trench are listed in Table 2. On the whole, in these four stations, the average concentrations of MCHO, PCHO, and TCHO all showed similar variation trends in different layers, with first decreasing, then increasing and then decreasing. Overall, the MCHO concentrations decreased gradually from the euphotic seawater to the bathypelagic seawater in open ocean (Pakulski and Benner, 1994), but in this study, the MCHO concentrations in the bathypelagic seawater were higher than those in the mesopelagic seawater at stations CTD03 and CTD04. The average concentrations of PCHO in the bathypelagic seawater at stations CTD01 and CTD02 were 11.2±4.3 μmol C/L and 9.3±4.2 μmol C/L, respectively, lower than the values of PCHO in the mesopelagic seawater, which were 18.0±6.7 μmol C/L and 14.7±6.6 μmol C/L, respectively. At stations CTD03 and CTD04, the average concentrations of PCHO in the bathypelagic seawater were 14.5±7.2 μmol C/L and 4.8±4.8 μmol C/L, respectively, higher than those in the mesopelagic layer, which were 7.6±5.2 μmol C/L and 3.2±3.2 μmol C/L, respectively. In the study area, the TCHO concentrations in the euphotic seawater at each station were the highest among all the water layers. At stations CTD01 and CTD02, the average concentrations of TCHO in the bathypelagic seawater were 21.9±7.7 μmol C/L and 14.6±3.0 μmol C/L, respectively, lower than those in the mesopelagic seawater, which were 28.1±6.5 μmol C/L and 24.1±6.4 μmol C/L, respectively. The results of TCHO were consistent with the variation trends of PCHO concentration in different layers.
|
The seawater samples collected from the five Dive stations in the Southern Yap Trench all obtained from the sediment-seawater interface, which belongs to the sediment overlying water. The concentrations of MCHO, PCHO, and TCHO are shown in Fig. 3. The stations Dive148 and Dive149 were in the abyss environment, and the rest of the stations were in the hadal environment. Among the five stations, the concentrations of MCHO ranged from 8.4 to 10.6 μmol C/L, which was close to the average concentration of MCHO in the deep seawater (below 1 000 m) of the four CTD stations. In the overlying water, the MCHO concentration reached the highest at station Dive148, and was the lowest at station Dive150. The PCHO concentrations of the seawater from the five Dive stations was not significantly different, ranging from 3.8 to 5.8 μmol C/L, with the maximum value at station Dive149 and the minimum value at station Dive 150. The concentrations of TCHO in the seawater samples of the five Dive stations ranged from 12.2 to 15.2 μmol C/L, with the highest value at station Dive148 and the lowest value at station Dive150.
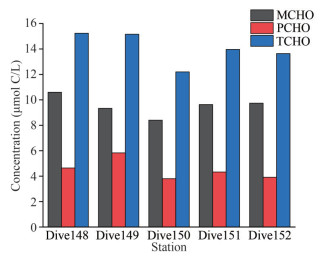
|
| Fig.3 Concentrations of MCHO, PCHO, and TCHO in the five Dive stations of the Southern Yap Trench |
The vertical changes of seawater temperature, salinity, and concentrations of DO and chlorophyll a in the Southern Yap Trench in the sampling period are shown in Fig. 4. The surface seawater temperature of the four CTD stations reached a maximum of 29.8 ℃, and dropped rapidly with depth. Below 2 000 m, the temperature was as low as 1–2 ℃ and remained stable. This kind of vertical variation pattern was similar to the temperature profiles of the seawater in the Northern Yap Trench (Guo et al., 2020). The vertical variation trends of salinity at the four CTD stations in the study area were similar, ranging from 33.3 to 34.7 from the surface seawater to the hadal zone. The salinity of the surface seawater in the Southern Yap Trench was significantly lower than that in the Northern Yap Trench. The average salinity of the Northern Yap Trench surface seawater was around 34.5, while the average value in the surface seawater of the study area was around 33.6. This difference was due to the fact that the Southern Yap Trench was closer to the equator, and the sampling time was closer to rain season, and then more precipitation occurred in the Southern Yap Trench compared to the Northern Yap Trench, which reduced the salinity of the surface seawater. Although there was some salinity difference of the surface water in the Southern and the Northern Yap Trench, the haloclines appeared in the 100–150-m water layer of the whole trench. Below 200 m, the salinity decreased and stabilized gradually in the study area. Liu et al. (2018) also confirmed the existence of thermocline and halocline in the water layer at 100–200 m in the Yap Trench, as well as a vertical gradient of potential temperature and salinity. The vertical profile patterns of the salinity in the Southern Yap Trench were almost the same as those in the Northern Yap Trench. Below the water depth of 200 m, the salinity decreased and tended to be stable. The vertical variation trends of DO concentrations in the seawater of the four CTD stations were very similar. The DO concentration was high in the surface water, and the oxygen minimum layer appeared at 300–1 000-m depth. Below the mesopelagic layer, the DO concentration increased slowly with depth. The variation profile patterns of salinity, temperature, and DO concentrations at the four CTD stations conformed to the characteristics of typical ocean water. In the 0–200-m euphotic layer, the Chl-a concentration in the seawater of station CTD01 was higher than those of the other three stations on the whole, and reached the highest at about 100-m water depth. At 200-m depth, the concentration of Chl a in the seawater decreased dramatically, and below 200 m, the concentration was below the detection limit. In the euphotic layer, the overall variations of Chl-a concentrations in the seawater shared similar patterns in the Northern (Guo et al., 2020) and the Southern Yap Trench, first decreasing, then increasing and then decreasing with depth. However, at the same depth, the Chl-a concentrations in the seawater of the Southern Yap Trench were higher than those in the Northern Yap Trench.
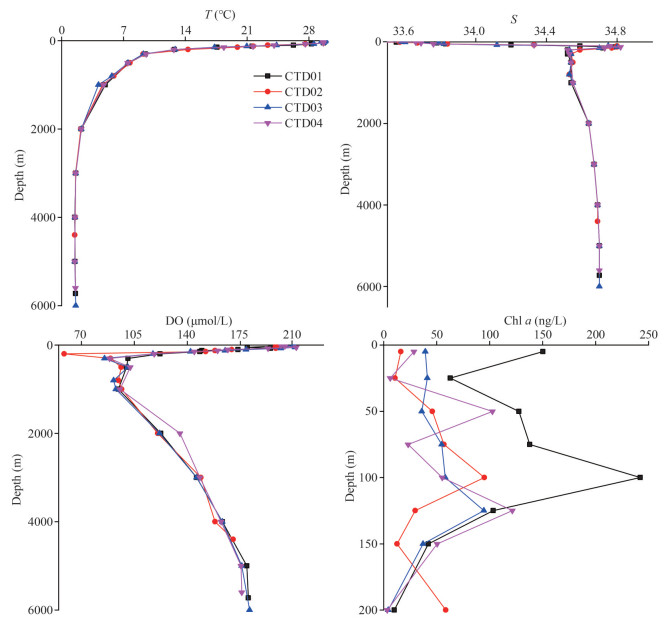
|
| Fig.4 Vertical variations of temperature (T), salinity (S), and concentrations of DO and Chl a in seawater of the Southern Yap Trench |
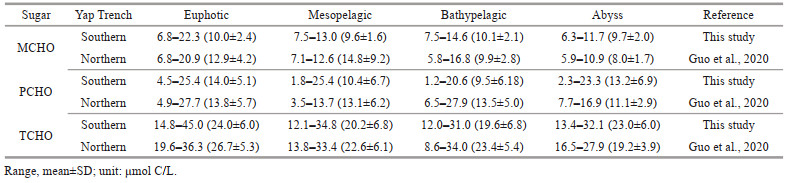
The comparison of the results of sugars between this study and previous studies in the seawater from different oceans, estuaries, and bays showed that the variation ranges of concentrations of dissolved sugars in the surface seawater of the Southern Yap Trench was similar to the results from oceans, and much lower than the values from estuaries and bays. In general, the concentration range of dissolved sugars in the surface water of the Yap Trench was consistent with the ranges in the surface water of other oceans. In addition, the summarized comparison of concentration ranges of MCHO, PCHO, and TCHO in the seawater of the CTD stations in the Southern and Northern Yap Trench were list in Table 3. Overall, the average concentrations of TCHO were slightly higher in the euphotic, mesopelagic, and bathypelagic layers of the Southern Yap Trench than those in the Northern Yap Trench. In the seawater of the abyssal layer, the average concentration of TCHO was slightly higher in the Northern Yap Trench.
At the four CTD stations in the Southern Yap Trench, the average concentration of MCHO in the euphotic layer was 11.6±4.6 μmol C/L, which was lower than the average concentration of MCHO in the euphotic layer in the Northern Yap Trench (Guo et al., 2020). In both the Southern and the Northern Yap Trench (Guo et al., 2020), the maximum concentrations of MCHO occurred in the euphotic seawater. The results of MCHO in this study were similar to those in the equatorial Pacific Ocean and the Gulf of Mexico (Pakulski and Benner, 1994; Hung et al., 2003), but the maximum concentration of PCHO in these two areas were 45.0 μmol C/L, higher than the maximum value of 36.3 μmol C/L in the seawater from the Northern Yap Trench. In the euphotic layer, the PCHO concentration range of 14.8–45.0 μmol C/L in the seawater of the Southern Yap Trench was close to the range of 19.6–36.3 μmol C/L in the seawater of the Northern Yap Trench. The average concentrations PCHO in the euphotic seawater of the two areas were very close, 24.0±6.0 μmol C/L and 26.7±5.3 μmol C/L, respectively. In the seawater of station CTD03, the maximum concentrations of MCHO, PCHO, and TCHO all appeared at the surface seawater, and in the seawater of stations CTD01 and CTD04, the maximum concentration of PCHO appeared at 200-m and 25-m depths, respectively. At these two stations, higher concentrations of Chl a were also detected in the seawater of the two depths. Chl-a concentration reflects the biomass of phytoplankton in seawater, and dissolved sugars are the direct products of phytoplankton photosynthesis (Benner et al., 1997; Kerhervé et al., 2002). This kind of consistence indicated the role of photosynthesis on the concentrations of sugars in the seawater. On the other hand, the thermocline and the halocline existed in similar water depths with high concentrations of dissolved sugars in the trench. To investigate the role of various seawater parameters on the vertical variations of the concentrations of dissolved sugars, the correlation analysis between the concentrations of dissolved sugars and the salinity, temperature, and DO concentrations of the seawater in the euphotic layer of the Southern Yap Trench was carried out, and the results are shown in Table 4.
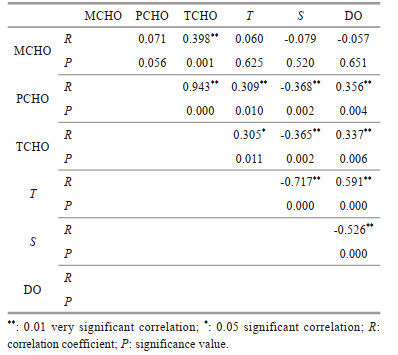
|
The results in Table 4 show that there was a significant negative correlation between the concentrations of PCHO and TCHO and the salinity in the seawater of the study area (R=-0.368, P= 0.002; R=-0.365, P=0.002). According to previous research, the nitrogen and phosphorus in the hourly precipitation in the Northwest Pacific Ocean could provide the corresponding sea areas with new productivity accounting for 0.013%–32.08% of the primary productivity, and the impact on the primary productivity should not be ignored (Lin et al., 2017). The primary productivity by extra nutrient input would lead to an increase in the concentrations of PCHO and TCHO. Therefore, due to the precipitation, it was reasonable to find negative correlations between the concentrations of PCHO and TCHO and the salinity in the euphotic seawater of the Southern Yap Trench. As a comparison, the correlation between PCHO and TCHO concentrations and salinity in the euphotic seawater of the Northern Yap Trench was insignificant. The results of the negative correlations in this study were consistent with that of Zhang et al. (2013) in the Jiaozhou Bay and that of Shi et al. (2017) in the East China Sea, indicating that the concentration of dissolved sugars in the seawater was controlled by a number of different factors. In marine environment, low salinity (high nutrient supply), sufficient light, and suitable temperature in the euphotic seawater provided favorable growth condition for phytoplankton, and the carbohydrate compounds formed by photosynthesis should be accumulated and released easily, causing high concentration of MCHO, PCHO, and TCHO in 0–200-m water layer (Ye et al., 2015). On the other hand, consumption processes of sugars always occurred in marine environment, including respiration of marine organisms (Walsh, 1965), photolysis of sugar into smaller molecules (Kovac et al., 1998), and etc., resulting in a decrease of the sugar concentrations in the seawater. The physical mixing of seawater was also an important factor controlling the concentration of dissolved sugars. For example, Shi et al. (2017) found that due to the strong vertical mixing of seawater in the marginal sea area, the high concentration region of carbohydrate in the East China Sea in winter was mainly distributed in the mixed layer, and the concentration decreased rapidly below the mixed layer. Hu et al. (2019) explored a section from the South Yellow Sea to the East China Sea. They found that the section located in the East China Sea was affected by the physical mixing of the Changjiang River water and the Taiwan Warm Current, and the distribution characteristics of MCHO, PCHO, and TCHO there were different from the section located in the South Yellow Sea. In addition, the adsorption and desorption of sugars by particulate matter also lead to a decrease or increase of the concentrations of dissolved sugars in seawater. All these processes could significantly change the concentrations of dissolved sugars in the euphotic seawater. In general, the variation trends of dissolved sugars in the seawater of the euphotic layers of the Southern and the Northern Yap Trench were similar, but the controlling factors on the trends were different.
4.2.2 Variation of the concentrations of carbohydrate from the mesopelagic to the abyssal seawater of the Southern Yap TrenchCompared to the euphotic layer, different environmental parameters such as temperature, DO, water pressure, light, and nutrients changed significantly, and the concentrations of dissolved sugars also changed significantly in the mesopelagic seawater of the Southern Yap Trench. The average concentrations of MCHO, PCHO, and TCHO in the mesopelagic seawater of the Southern Yap Trench were 9.6±1.6 μmol C/L, 10.4±6.7 μmol C/L, and 20.2±6.8 μmol C/L, with a decreasing trend from the euphotic layer. This variation trend was closely related to a variety of biogeochemical processes in the ocean. The concentrations of dissolved sugars in the mesopelagic seawater of the Yap Trench was mainly controlled by the following processes: microbial respiration consuming MCHO, PCHO being decomposed to MCHO, dead plankton sinking and decomposing, dissolution and releasing of granular sugars, adsorption and desorption processes, and etc. Without light, there were no sugars produced by phytoplankton photosynthesis in the mesopelagic seawater of the Yap Trench, but sugar consumption still existed. Carbohydrates are important sources of carbon and energy for heterotrophic microorganisms (Nunoura et al., 2015). While consuming carbohydrates, microorganisms also consumed a large amount of dissolved oxygen, so that in the mesopelagic seawater in the Southern Yap Trench, the concentration of dissolved oxygen decreased remarkably. Additional to oxidation of sugars, the processes such as microbial growth, diagenetic transformation and mineralization, the oxidative decomposition of organic matter and the respiration of marine organisms all lead to the formation of the oxygen minimum layer in 500–1 000-m water depth (Wishner et al., 1995). In the ocean, the combined effect of the processes reached the highest degree at 1 000-m depth (Li et al., 2020). The direct consumption of MCHO by microorganisms reduced its concentration significantly in the mesopelagic seawater, so the minimum concentration of MCHO appeared in the layer of all the four stations. However, the average concentration of MCHO decreased by only 0.4 μmol C/L from the euphotic seawater to the mesopelagic seawater, indicating that there was a source of MCHO in the mesopelagic seawater in the study area. Through correlation analysis, it was found that the concentrations of PCHO and TCHO were positively correlated with the DO concentrations in the mesopelagic seawater in the Southern Yap Trench (Table 4). However, although MCHO was directly consumed by marine microbial respiration, the correlation between MCHO concentrations and DO concentrations in the mesopelagic seawater of the Southern Yap Trench seawater was not significant. Our results also showed that the DO concentrations in the mesopelagic seawater in the study area was 50% less than the euphotic layer, but the TCHO concentration was only reduced by less than 16%, indicating that TCHO was supplemented to a certain extent in the mesopelagic layer. In fact, in addition to consuming and utilizing MCHO, the microbial processes in the mesopelagic seawater could also decompose the sinking plankton remains and faeces to release sugars, resulting in the production of more PCHO. Studies on the decomposition process of granular organic matter showed that granular carbohydrates were preferentially decomposed by bacteria and then dissolved in seawater (Shi et al., 2017), thereby increasing the concentration of PCHO in the seawater. After that, the hydrolysis of PCHO increased the MCHO concentrations in the seawater. Compared with the euphotic layer, the results of this study showed that the average concentration of PCHO in the mesopelagic seawater decreased more than 25.7%, suggesting that PCHO was an important source of MCHO in the layer. Since the decrease of MCHO concentration was much less than that of PCHO concentration, the decreasing trend of TCHO concentration was not as obvious as that of PCHO in the mesopelagic layer of the Southern Yap Trench.
In different CTD stations of the study area, the respiration of microorganisms, the decomposition of organic matter, and the hydrolysis of polysaccharides had different strengths. The combined effect of these processes resulted in significant differences in the forms and concentrations of dissolved sugars in the mesopelagic seawater of the stations. For example, with increasing depth, the average concentration of MCHO at station CTD04 increased from 9.1±1.4 μmol C/L in the euphotic seawater to 11.6±1.3 μmol C/L in the mesopelagic seawater, while the average concentration of PCHO decreased from 9.9±4.0 μmol C/L to 3.2±1.6 μmol C/L, indicating that more PCHO in the mesopelagic seawater at this station formed MCHO through hydrolysis. At stations CTD01 and CTD02, the average concentration of PCHO increased from 15.5±4.3 μmol C/L and 14.5±2.7 μmol C/L in the euphotic seawater to 18.0±6.7 μmol C/L and 14.7±6.6 μmol C/L in the mesopelagic seawater, respectively, indicating that the process of releasing granular polysaccharides into seawater to form PCHO was more active at these two stations. On the other hand, previous studies showed that in 300–1 000-m seawater, the carbohydrates were mainly in insoluble state, and the dissolved carbohydrates could be adsorbed on the surface of natural debris, and could enter the seawater through desorption process with the sedimentation of debris, becoming a source of dissolved sugars in the mesopelagic seawater (Zhang et al., 2004). Compared with the euphotic seawater, the combined effect of respiration, the decomposition of biological particles, the hydrolysis of polysaccharides, the adsorption and desorption of particles, and etc., resulting in a decrease in the concentration of dissolved sugars in the mesopelagic seawater of the Southern Yap Trench. In general, the complex distribution patterns of PCHO concentrations from the euphotic layer to the mesopelagic seawater in the Southern Yap Trench were similar to those in the Northern Yap Trench (Guo et al., 2020). The concentrations of TCHO in the mesopelagic seawater was lower than those in the euphotic layer and the bathypelagic seawater, and this variation trend was consistent with the trend of sugars in the Northern Yap Trench (Guo et al., 2020). The consistency of the trends indicated that the seawater from the euphotic layer to the mesopelagic layer of the Northern and the Southern Yap Trench could be considered as a whole.
In the bathypelagic seawater of the Southern Yap Trench, the DO concentrations gradually recovered with the water depth from the mesopelagic seawater, indicating that the respiration in this layer weakened. In the seawater of this layer, in addition to various biological and chemical processes, current conditions and funnel effects as the factors affecting the concentrations and forms of dissolved sugars in the seawater should not be ignored. The changes of MCHO concentrations in the bathypelagic seawater of the Southern Yap Trench were mainly affected by microbial respiration and polysaccharide hydrolysis. The average concentration of MCHO from the mesopelagic seawater to the bathypelagic seawater at stations CTD01 and CTD03 increased a little from 10.1±1.2 μmol C/L and 8.1±0.9 μmol C/L to 10.7±3.4 μmol C/L and 10.1±2.2 μmol C/L, indicating that the polysaccharide hydrolysis in these stations became the main factor affecting the MCHO concentrations in the bathypelagic seawater. In stations CTD01 and CTD04, the average concentration of MCHO showed opposite trend with stations CTD01 and CTD02 from the mesopelagic seawater to the bathypelagic seawater, decreasing a little from 9.4±0.6 μmol C/L and 11.6±1.3 μmol C/L to 8.1±0.3 μmol C/L and 11.3±0.9 μmol C/L, indicating that the respiration of microorganisms surpassed the hydrolysis of polysaccharides in the bathypelagic layer. Compared to the mesopelagic seawater, average PCHO concentrations at stations CTD01 and CTD02 decreased from 18.0±6.7 μmol C/L and 14.7±6.6 μmol C/L to 11.2±4.3 μmol C/L and 9.3±4.2 μmol C/L, and the average concentration of TCHO decreased from 28.1±6.5 μmol C/L and 24.1±6.4 μmol C/L to 21.9±7.7 μmol C/L and 14.6±3.0 μmol C/L in the bathypelagic seawater. The decrease of PCHO and TCHO concentrations indicated that the rate of hydrolysis of PCHO to MCHO was faster at these two stations, exceeding the rate at which the particulate sugars were broken down to PCHO. However, at stations CTD03 and CTD04, the average PCHO concentrations increased from 7.6±5.2 μmol C/L and 3.2±1.6 μmol C/L in the mesopelagic seawater to 14.5±7.2 μmol C/L and 4.8±4.8 μmol C/L in the bathypelagic seawater, and the average TCHO concentrations increased from 16.8±6.1 μmol C/L and 14.8±0.8 μmol C/L in the mesopelagic seawater to 24.7±9.0 μmol C/L and 16.1±5.7 μmol C/L in the bathypelagic seawater, indicating that there was a large release of PCHO in the bathypelagic seawater at these two stations. Previous study suggested that in the bathypelagic seawater, with the increase of water depth, the shells of calcareous organisms and calcareous particles kept dissolving, so that particulate polysaccharides existed in these particle were continuously released to form PCHO (Guo et al., 2020). The concentrations of PCHO and TCHO in the seawater increased if PCHO hydrolysis to MCHO was slow at low water temperature. In the bathypelagic layer and below, the "V" -shaped structure of the Yap Trench was conducive to the vertical transport of organic matter (Guo et al., 2020), forming a funnel effect and promoting the migration of particulate organic matter from the mesopelagic seawater to deeper seawater, and then provided more source of polysaccharide below the mesopelagic layer. Previous research showing that a small branch of the Lower Circumpolar Water flow through a part of the Yap Trench (Johnson and Toole, 1993). Therefore, it was possible that the bathypelagic seawater in stations CTD03 and CTD04 were affected by the seawater around the Antarctic with higher TCHO concentrations (Liu et al., 2020), and then the concentrations of dissolved carbohydrates increased in the bathypelagic layer. The low temperature in the bathypelagic seawater also limited the activities of marine microorganisms, reduced their energy consumption, and then increased the concentrations of carbohydrates and DO concentrations there. Low microbial activity might be another reason for detecting higher concentrations of PCHO and TCHO in the bathypelagic seawater of the two stations. Similar to the Northern Yap Trench (Guo et al., 2020), the changes of sugar concentrations in the bathypelagic seawater of the Southern Yap Trench were the results of comprehensive effects of different physical, chemical, and biological factors.
In the abyssal seawater (4 000–6 000 m) of the Southern Yap Trench, the DO concentrations kept increasing with depth, and the average concentration of MCHO decreased slightly from 10.1±2.1 μmol C/L in the bathypelagic seawater to 9.7±2.0 μmol C/L in the abyssal layer. Unlike MCHO, the average concentrations of PCHO and TCHO increased from 10.1±2.1 μmol C/L and 19.6±6.8 μmol C/L in the bathypelagic layer to 13.2±6.9 μmol C/L and 23.0±6.0 μmol C/L in the abyssal layer. Our results show that with the continuous consumption of sugars in the sinking process from the bathypelagic layer to the abyss, the hydrolysis of PCHO was further weakened by the environmental low temperature, and the respiration of microorganisms preferentially consumed the dissolved MCHO, which caused decreasing trend of the MCHO concentrations and increasing trend of PCHO concentrations in the abyssal seawater as a whole. On the other hand, with the further decrease of seawater temperature in the abyss, the weakening of microbial respiration significantly reduced the consumption of MCHO, so that the concentration of MCHO decrease insignificantly compared to the bathypelagic seawater. Our results show that the MCHO concentration increased and the PCHO concentration decreased in the deepest seawater at stations CTD01 and CTD02, indicating that there might be a conversion of PCHO to MCHO in the water layer. At stations CTD01, CTD03, and CTD04, the bottom seawater was close to the sediment-seawater interface. The TCHO concentrations were slightly elevated in the seawater samples from stations CTD01 and CTD04, attributed to sediment resuspension caused by vertical mixing of seawater, which released some sugars in the sediments into the seawater (Arnosti and Holmer, 1999). Witter and Luther III (2002) also observed an increase in the concentration of dissolved sugars at the sediment-seawater interface in the Delaware Estuary. The phenomenon of elevated TCHO concentrations in seawater near the sediment-seawater interface was also reported in the abyssal water close to the sediment-seawater interface of the Northern Yap Trench (Guo et al., 2020). On the whole, the concentrations of dissolved sugars in the abyssal seawater were the results of the combined effects of respiration, deep sea water current, biological activities, transformation of granular sugars, sediment resuspension and etc.
4.2.3 Concentration of dissolved carbohydrates in the seawater from the sediment-seawater interface of the Southern Yap TrenchThe seawater samples from the five Dive stations in the Southern Yap Trench were all from the seawater-sediment interface. The average concentrations of MCHO, PCHO, and TCHO in the seawater of these stations were 9.5±0.8 μmol C/L, 4.5±1.2 μmol C/L, and 14.1±0.8 μmol C/L, respectively. Compared with the seawater at the sediment-seawater interface of the Northern Yap Trench, the MCHO concentration range was similar, while the PCHO concentration was lower as a whole in the seawater samples. Among the five stations, stations Dive150, Dive151, and Dive152 were located in the hadal zone, and their MCHO, PCHO, and TCHO concentrations of the seawater samples were lower than those of stations Dive148 and Dive149 in the abyss, indicating that dissolved sugars were further consumed in the hadal seawater, and the concentrations of sugars further decreased there. Different factors, such as steep slopes, narrow landforms, submarine earthquakes, landslides, as well as input sinking and resuspension of organic matter, all impacted the concentrations of dissolved sugars in the hadal seawater. The "V" -shaped structure of the hadal zone in the Yap Trench is steeper, and the suspended organic matter from the slope might affect the biogeochemical cycle of the entire trench (Nunoura et al., 2015), which in turn affected the concentrations of dissolved sugars in the overlying water of the sediment-seawater interface. The average concentration of TCHO in the hadal seawater in the Southern Yap Trench was 13.3±0.94 μmol C/L, lower than the value in the hadal seawater of the Northern Yap Trench. The Southern Yap Trench is formed by the subduction of the Caroline Plate under the Philippine Plate (Seno et al., 1993), and the Northern Yap Trench is formed by the subduction of the Pacific Plate and the Caroline Plate under the Philippine Plate (Altis, 1999). The fewer plates in the south part of the trench would cause less geological activities than that in the north, and then less sediment was produced in the abyss and hadal zone by the activities in the Southern Yap Trench. The reduced frequency of geological activities also reduced sediment resuspension and the release of sugars from sediment to seawater. The average concentration of MCHO was higher than that of PCHO of the seawater from the Dive stations in the Southern Yap Trench, while the opposite result appeared the Northern Yap Trench. Previous studies showed that suspended organic matter associated with earthquake-induced seafloor deformation (landslides and turbulent sediments) affected the microbial cell abundance and composition of the bathypelagic microbial communities in the Japan Trench (Kawagucci et al., 2013). Fewer earthquakes in the Southern Yap Trench might also reduce microbial abundance and MCHO consumption, resulting in higher concentrations of MCHO than PCHO in the hadal seawater there. On the other side, Zhai et al. (2022) studied the bacterial communities developed on different metal surface at 5 772 m undersea in the northern trench. They found the existence of heterotrophic Protebacteria, Stenotrophomonas, Burkholderia-Caballeronia-Paraburkholderia, and Achromobacter as sugar consumer, and autotrophic Sulfurimonas as sugar producer in the sediment-seawater interface. With less geological activities in the southern trench than those in the northern trench, it is possible that less energy could be used for assimilation of the autotrophic bacteria, and then the source of sugar from local bacterial production should be less, providing another possible explanation of lower TCHO concentration in the Southern Yap Trench than that in the Northern Yap Trench.
It is interested that concentration of PCHO in the hadal zone of the trench might significantly affect its concentration in the abyss of the trench. From Table 3, in the abyss of the southern trench, the concentration of PCHO was lower than that of the northern trench. As a comparison, the concentrations of PCHO in the euphotic, mesopelagic, and bathypelagic layer were all higher in the southern trench. Liu et al. (2020) pointed out that the Northern and Southern Yap Trench shared similar properties of water mass in the abyss. The abyssal circulation of the trench was linked to the Lower Circumpolar Water (LCPW). In the hadal zone, the water in the northern and southern trench was considered as isolated local water. In abyssal and hadal zone below 4 500 m, the diapycnal mixing increased with depth and then the water stratification weakened in the deep abyssal layer and hadal zone. In the hadal zone of the southern trench, the PCHO concentration was lower than that of the northern trench. Although the water mass in the abyss of the whole trench was similar, the mixing process of the abyssal and hadal seawater might cause lower PCHO concentration in the abyss of the Southern Yap Trench.
4.3 The ratio of MCHO/TCHO and PCHO/TCHO in the seawater from the Southern Yap TrenchIn the seawater of the Southern Yap Trench, the vertical variation profiles of the concentration ratios of MCHO to TCHO and concentrations of PCHO to TCHO in the seawater samples were different among the four CTD stations (Fig. 5). Among the 69 seawater samples from the stations, 46 samples had PCHO/TCHO values above 0.50. Overall, the mean values for MCHO/TCHO and PCHO/TCHO were 0.48 and 0.52, respectively. From the average of PCHO/TCHO and MCHO/TCHO, PCHO was considered as the major form of dissolved carbohydrates in the Southern Yap Trench. This conclusion was consistent with the conclusion that the major form of dissolved sugar was PCHO in the Northern Yap Trench, indicating that PCHO occupied the majority of dissolved sugar in the seawater of the whole trench (Guo et al., 2020).
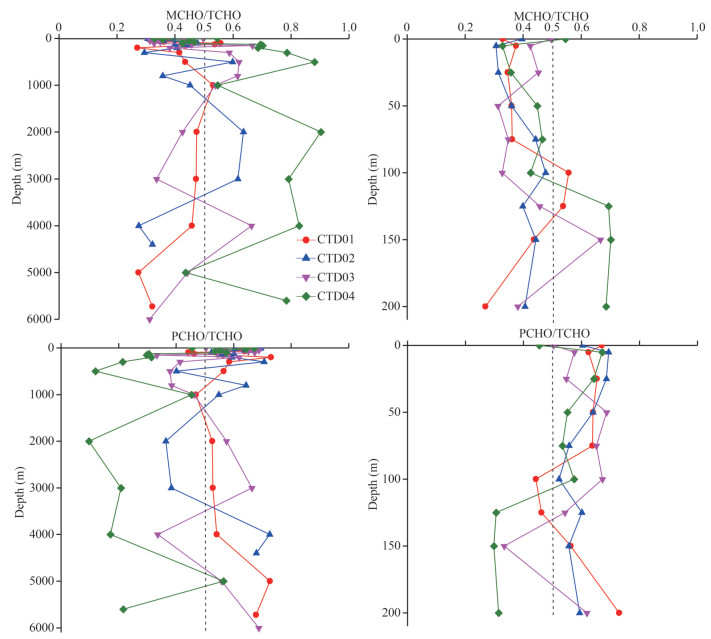
|
| Fig.5 Vertical variations of MCHO/TCHO and PCHO/TCHO at the four CTD stations of the Southern Yap Trench |
Among the vertical profiles of MCHO/TCHO and PCHO/TCHO, the profiles at stations CTD01, CTD02, and CTD03 were more complex. In the euphotic seawater of station CTD01, the average value of MCHO/TCHO was smaller than the average value of PCHO/TCHO, and PCHO was the major faction of TCHO of the seawater. PCHO was a product of phytoplankton photosynthesis and a major component of their cell wall (Myklestad and Børsheim, 2007). In the euphotic layer, after the death of phytoplankton, PCHO was released into the seawater, making PCHO the main component of TCHO. However, in the seawater below the thermocline and halocline, the values of MCHO/TCHO and PCHO/TCHO became close, indicating that with the weakening of photosynthesis, PCHO began to be hydrolyzed to MCHO, making its proportion in the total TCHO decreased. In general, the MCHO/TCHO values in the mesopelagic seawater of the study area were higher than that of PCHO/TCHO, indicating that MCHO contributed more to the TCHO in the layer, and suggesting that more PCHO was converted to MCHO in the seawater. From the bathypelagic layer to the abyssal layer, the PCHO/TCHO values of the seawater at stations CTD01 and CTD03 were higher than those of MCHO/TCHO, indicating that PCHO was the major dissolved sugar in the seawater below 1 000-m depth at these two stations. As a comparison, at stations CTD02 and CTD04, most of the PCHO/TCHO values in the seawater below mesopelagic layer were lower than those of MCHO/TCHO, indicating that the major form of dissolved sugars in the bathypelagic and abyssal seawater was MCHO. In theory, MCHO can be directly used by microorganisms, so it is easier to be degraded. On the other hand, PCHO can be hydrolyzed to MCHO in seawater by heterotrophic bacteria (Arnosti et al., 2021). The influence of these two processes on the concentrations of PCHO and MCHO in seawater varied with marine environmental conditions, especially the activities of microorganisms. Therefore, the MCHO/TCHO and PCHO/TCHO might have different variation trends in different stations. Different from the four CTD stations, the MCHO/TCHO values in the seawater samples from the five Dive stations ranged from 0.62 to 0.71, far exceeding 0.5. Since the abundance of microorganisms in sediment was much higher than that in seawater and had the ability to convert more PCHO to MCHO, and the resuspension of sediment also promoted to release more MCHO into the overlying water, the proportion of MCHO in TCHO increased significantly in the seawater samples from all the Dive stations.
5 CONCLUSIONThis study focused on the vertical variation characteristics of dissolved carbohydrates of the seawater in the Southern Yap Trench. The results showed that the concentrations of MCHO, PCHO, and TCHO in the seawater of the Southern Yap Trench ranged from 6.3 to 22.3 μmol C/L, 1.1 to 25.4 μmol C/L, and 12.1 to 44.9 μmol C/L, respectively. PCHO was the major form of dissolved carbohydrates in the seawater of the study area. In general, the vertical variation patterns of the dissolved sugar concentrations in the seawater of the Southern Yap Trench were similar to those in the Northern Yap Trench, showing decreasing trends with depth. The photosynthesis and respiration of plankton were the key factors affecting the seawater sugars in the euphotic layer of the Southern Yap Trench. In the mesopelagic layer, microbial respiration, hydrolysis of polysaccharides, dissolution and release from particulate sugars, adsorption and desorption processes were the control factors on the concentration and morphology of the dissolved sugars in the seawater. From the bathypelagic layer to the abyssal layer of the Southern Yap Trench, some physical processes including deep ocean currents, mixing of water masses, and funnel effect of the trench significantly affected the concentrations and morphology of the dissolved sugars. The concentrations of MCHO, PCHO, and TCHO in the overlying water of the five Dive stations in the Southern Yap Trench ranged from 8.40–10.60 μmol C/L, 3.80–5.82 μmol C/L, and 12.20–15.24 μmol C/L, respectively. MCHO was the majority of TCHO of the seawater at the sediment-seawater interface of the study area. The activities of microorganisms in sediments and the sediment resuspension were the important factors affecting the concentrations and morphology of dissolved sugars in the overlying seawater of the sediment-seawater interface in the abyss and hadal zone of the Southern Yap Trench.
6 DATA AVAILABILITY STATEMENTData sets generated during this study are available to corresponding authors upon reasonable request.
7 ACKNOWLEDGMENTThe authors would like to thank the crew and scientists aboard R/V Xiangyanghong 09 and Jiaolong submersibles. The authors also appreciate the members in the Marine Interface Chemistry Lab of the Ocean University of China.
Altis S. 1999. Origin and tectonic evolution of the Caroline Ridge and the Sorol Trough, western tropical Pacific, from admittance and a tectonic modeling analysis. Tectonophysics, 313(3): 271-292.
DOI:10.1016/S0040-1951(99)00204-8 |
Arnosti C, Holmer M. 1999. Carbohydrate dynamics and contributions to the carbon budget of an organic-rich coastal sediment. Geochimica et Cosmochimica Acta, 63(3-4): 393-403.
DOI:10.1016/S0016-7037(99)00076-9 |
Arnosti C, Wietz M, Brinkhoff T, et al. 2021. The biogeochemistry of marine polysaccharides: sources, inventories, and bacterial drivers of the carbohydrate cycle. Annual Review of Marine Science, 13: 81-108.
DOI:10.1146/annurev-marine-032020-012810 |
Benner R, Biddanda B, Black B, et al. 1997. Abundance, size distribution, and stable carbon and nitrogen isotopic compositions of marine organic matter isolated by tangential-flow ultrafiltration. Marine Chemistry, 57(3-4): 243-263.
DOI:10.1016/S0304-4203(97)00013-3 |
Bhosle N B, Bhaskar P V, Ramachandran S. 1998. Abundance of dissolved polysaccharides in the oxygen minimum layer of the Northern Indian Ocean. Marine Chemistry, 63(1-2): 171-182.
DOI:10.1016/S0304-4203(98)00061-9 |
Burney C M, Johnson K M, Lavoie D M, et al. 1979. Dissolved carbohydrate and microbial ATP in the North Atlantic: concentrations and interactions. Deep-Sea Research Part A. Oceanographic Research Papers, 26(11): 1267-1290.
DOI:10.1016/0198-0149(79)90068-2 |
Carlson C A, Ducklow H W, Michaels A F. 1994. Annual flux of dissolved organic carbon from the euphotic zone in the northwestern Sargasso Sea. Nature, 371(6496): 405-408.
DOI:10.1038/371405a0 |
Fujiwara T, Tamura C, Nishizawa A, et al. 2000. Morphology and tectonics of the Yap Trench. Marine Geophysical Researches, 21(1-2): 69-86.
DOI:10.1023/A:1004781927661 |
Guo C, Sun C, Yang G P, et al. 2020. Vertical variations of dissolved carbohydrates in the North Yap Trench. Journal of Oceanology and Limnology, 38(3): 606-618.
DOI:10.1007/s00343-020-9018-8 |
Guo C Y, Yang Z, Chen J F, et al. 2018. A preliminary study on the food resources and trophic levels of the benthic community in the Yap Trench based on stable carbon and nitrogen isotopes. Acta Oceanologica Sinica, 40(10): 51-60.
(in Chinese with English abstract) |
Hawkins J, Batiza R. 1977. Metamorphic rocks of the Yap arc-trench system. Earth and Planetary Science Letters, 37(2): 216-229.
DOI:10.1016/0012-821X(77)90166-2 |
Görs S, Schumann R, Häubner N, et al. 2007. Fungal and algal biomass in biofilms on artificial surfaces quantified by ergosterol and chlorophyll a as biomarkers. International Biodeterioration and Biodegradation, 60(1): 50-59.
DOI:10.1016/j.ibiod.2006.10.003 |
He Z, Wang Q, Yang G P, et al. 2015. Spatiotemporal variation characteristics and related affecting factors of dissolved carbohydrates in the East China Sea. Continental Shelf Research, 108: 12-24.
DOI:10.1016/j.csr.2015.08.002 |
Hu C, Chen Y, Yang G P, et al. 2019. Study on the distribution of dissolved carbohydrates in the South Yellow Sea and the East China Sea during spring. Marine Environmental Science, 38(6): 848-855.
(in Chinese with English abstract) |
Huang Y, Sun C, Yang G P, et al. 2020. Geochemical characteristics of hadal sediment in the northern Yap Trench. Journal of Oceanology and Limnology, 38(3): 650-664.
DOI:10.1007/s00343-019-9010-3 |
Hung C C, Guo L D, Santschi P H, et al. 2003. Distributions of carbohydrate species in the Gulf of Mexico. Marine Chemistry, 81(3-4): 119-135.
DOI:10.1016/S0304-4203(03)00012-4 |
Hung C C, Tang D G, Warnken K W, et al. 2001. Distributions of carbohydrates, including uronic acids, in estuarine waters of Galveston Bay. Marine Chemistry, 73(3-4): 305-318.
DOI:10.1016/S0304-4203(00)00114-6 |
Hung C C, Warnken K W, Santschi P H. 2005. A seasonal survey of carbohydrates and uronic acids in the Trinity River, Texas. Organic Geochemistry, 36(3): 463-474.
DOI:10.1016/j.orggeochem.2004.09.004 |
Hung J J, Lin P L, Liu K K. 2000. Dissolved and particulate organic carbon in the southern East China Sea. Continental Shelf Research, 20(4-5): 545-569.
DOI:10.1016/S0278-4343(99)00085-0 |
Ji C X, Yang G P, Chen Y, et al. 2019. Distribution, degradation and bioavailability of dissolved organic matter in the East China Sea. Biogeochemistry, 142(2): 189-207.
DOI:10.1007/s10533-018-0529-8 |
Johnson G C, Toole J M. 1993. Flow of deep and bottom waters in the Pacific at 10°N. Deep-Sea Research Part Ⅰ: Oceanographic Research Papers, 40(2): 371-394.
DOI:10.1016/0967-0637(93)90009-R |
Kawagucci S, Ueno Y, Takai K, et al. 2013. Geochemical origin of hydrothermal fluid methane in sediment-associated fields and its relevance to the geographical distribution of whole hydrothermal circulation. Chemical Geology, 339: 213-225.
DOI:10.1016/j.chemgeo.2012.05.003 |
Kawasaki N, Benner R. 2006. Bacterial release of dissolved organic matter during cell growth and decline: molecular origin and composition. Limnology and Oceanography, 51(5): 2170-2180.
DOI:10.4319/lo.2006.51.5.2170 |
Kerhervé P, Buscail R, Gadel F, et al. 2002. Neutral monosaccharides in surface sediments of the northwestern Mediterranean Sea. Organic Geochemistry, 33(4): 421-435.
DOI:10.1016/S0146-6380(02)00003-7 |
Khodse V B, Fernandes L, Gopalkrishna V V, et al. 2007. Distribution and seasonal variation of concentrations of particulate carbohydrates and uronic acids in the northern Indian Ocean. Marine Chemistry, 103(3-4): 327-346.
DOI:10.1016/j.marchem.2006.10.003 |
Kovac N, Faganeli J, Sket B, et al. 1998. Characterization of macroaggregates and photodegradation of their water soluble fraction. Organic Geochemistry, 29(5-7): 1623-1634.
DOI:10.1016/S0146-6380(98)00178-8 |
Li Y N, Chen H T, Gu W Y, et al. 2020. The vertical distribution of nutrients in the Challenge Deep of the Mariana Trench. Periodical of Ocean University of China, 50(1): 74-81.
(in Chinese with English abstract) |
Lin J R, Qi J H, Xie D D, et al. 2017. The concentrations and wet depositions fluxes of inorganic ions in oceanic precipitation—study on precipitation over the China Sea and Northwest Pacific Ocean. China Environmental Science, 37(5): 1706-1715.
(in Chinese with English abstract) |
Liu X H, Liu Y Z, Cao W, et al. 2022. Flow pathways of abyssal water in the Yap Trench and adjacent channels and basins. Frontiers in Marine Science, 9: 910941.
DOI:10.3389/fmars.2022.910941 |
Liu Y Z, Liu X H, Lv X Q, et al. 2018. Watermass properties and deep currents in the northern Yap Trench observed by the Submersible Jiaolong system. Deep-Sea Research Part Ⅰ: Oceanographic Research Papers, 139: 27-42.
DOI:10.1016/j.dsr.2018.06.001 |
Liu Y H, Liu X Z, Lv X, et al. 2020. Water characteristics of abyssal and hadal zones in the southern Yap Trench observed with the submersible Jiaolong. Journal of Oceanology and Limnology, 38(3): 593-605.
DOI:10.1007/s00343-019-8368-6 |
Myklestad S M, Børsheim K Y. 2007. Dynamics of carbohydrates in the Norwegian Sea inferred from monthly profiles collected during 3 years at 66°N, 2°E. Marine Chemistry, 107(4): 475-485.
DOI:10.1016/j.marchem.2007.09.002 |
Myklestad S M, Skånøy E, Hestmann S. 1997. A sensitive and rapid method for analysis of dissolved mono- and polysaccharides in seawater. Marine Chemistry, 56(3-4): 279-286.
DOI:10.1016/S0304-4203(96)00074-6 |
Nunoura T, Takaki Y, Hirai M, et al. 2015. Hadal biosphere: insight into the microbial ecosystem in the deepest ocean on Earth. Proceedings of the National Academy of Sciences of the United States of America, 112(11): E1230-E1236.
DOI:10.1073/pnas.1421816112 |
Pakulski J D, Benner R. 1994. Abundance and distribution of carbohydrates in the ocean. Limnology and Oceanography, 39(4): 930-940.
DOI:10.4319/lo.1994.39.4.0930 |
Sato T, Kasahara J, Katao H, et al. 1997. Seismic observations at the Yap Islands and the northern Yap Trench. Tectonophysics, 271(3-4): 285-294.
DOI:10.1016/S0040-1951(96)00251-X |
Schlitzer R. 2018. Ocean Data View. https://odv.awi.de/.
|
Seno T, Stein S, Gripp A E. 1993. A model for the motion of the Philippine Sea plate consistent with NUVEL-1 and geological data. Journal of Geophysical Research: Solid Earth, 98(B10): 17941-17948.
DOI:10.1029/93JB00782 |
Shi D, Yang G P, Sun Y. 2017. Distribution of dissolved carbohydrates and its influencing factors in the East China Sea during winter. Marine Environmental Science, 36(4): 481-487.
(in Chinese with English abstract) |
Thornton D C O. 2014. Dissolved organic matter (DOM) release by phytoplankton in the contemporary and future ocean. European Journal of Phycology, 49(1): 20-46.
DOI:10.1080/09670262.2013.875596 |
Walsh G E. 1965. Studies on dissolved carbohydrate in Cape Cod waters. Ⅱ. Diurnal fluctuation in oyster pond. Limnology and Oceanography, 10(4): 577-582.
DOI:10.4319/lo.1965.10.4.0577 |
Wang D L, Henrichs S M, Guo L D. 2006. Distributions of nutrients, dissolved organic carbon and carbohydrates in the western Arctic Ocean. Continental Shelf Research, 26(14): 1654-1667.
DOI:10.1016/j.csr.2006.05.001 |
Witter A E, Luther Ⅲ G W. 2002. Spectrophotometric measurement of seawater carbohydrate concentrations in neritic and oceanic waters from the U. S. Middle Atlantic Bight and the Delaware estuary. Marine Chemistry, 77(2-3): 143-156.
DOI:10.1016/S0304-4203(01)00083-4 |
Wishner K F, Ashjian C J, Gelfman C, et al. 1995. Pelagic and benthic ecology of the lower interface of the Eastern Tropical Pacific oxygen minimum zone. Deep-Sea Research, Part Ⅰ: Oceanographic Research Papers, 42(1): 93-115.
DOI:10.1016/0967-0637(94)00021-J |
Yan Y, Sun C, Huang Y, et al. 2020. Distribution characteristics of lipids in hadal sediment in the Yap Trench. Journal of Oceanology and Limnology, 38(3): 634-649.
DOI:10.1007/s00343-019-8120-2 |
Ye L L, Wu X D, Kong F X, et al. 2015. Sources of dissolved organic carbon and the bioavailability of dissolved carbohydrates in the tributaries of Lake Taihu. Environmental Science, 36(3): 914-921.
(in Chinese with English abstract) |
Zeppenfeld S, van Pinxteren M, van Pinxteren D, et al. 2021. Aerosol marine primary carbohydrates and atmospheric transformation in the western Antarctic peninsula. ACS Earth and Space Chemistry, 5(5): 1032-1047.
DOI:10.1021/acsearthspacechem.0c00351 |
Zhai X F, Cao W, Zhang Y M, et al. 2022. Study on the bacterial communities of the biofilms on titanium, aluminum, and copper alloys at 5, 772 m undersea in Yap Trench. Frontiers in Microbiology, 13: 831984.
DOI:10.3389/fmicb.2022.831984 |
Zhang S, Fang Z X. 1991. Dissolved carbohydrate in Daya bay waters. Tropic Oceanology, 10(4): 89-93.
(in Chinese with English abstract) |
Zhang Y P, Yang G P, Lu X L, et al. 2013. Distributions of dissolved monosaccharides and polysaccharides in the surface microlayer and surface water of the Jiaozhou Bay and its adjacent area. Continental Shelf Research, 63: 85-93.
DOI:10.1016/j.csr.2013.05.002 |
Zhang Z B, Liu L S. 2004. Marine Chemistry. Shandong Education Press, Ji'nan, China. p. 185-203.
(in Chinese)
|
 2023, Vol. 41
2023, Vol. 41