Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HU Xin, LI Jing, WANG Juan, YIN Li, SHI Kaipian, HUANG Heyong, ZHANG Yong, LI Shiyin
- Characterization and stability of sedimentary colloids in different ecology regions in Taihu Lake
- Journal of Oceanology and Limnology, 41(6): 2146-2159
- http://dx.doi.org/10.1007/s00343-022-2254-3
Article History
- Received Jul. 7, 2022
- accepted in principle Aug. 15, 2022
- accepted for publication Sep. 19, 2022
2 Analysis and Testing Center of Nanjing Normal University, Nanjing Normal University, Nanjing 210023, China;
3 Jiangsu Center for Collaborative Innovation in Geographical Information Resource Development and Application, Nanjing 210023, China;
4 Department of Geological Sciences, University of Alabama, Tuscaloosa AL 35487, USA;
5 Jiangsu Open Laboratory of Large-scale Scientific Instruments, Nanjing Normal University, Nanjing 210023, China
Colloids exist in aquatic environments such as freshwater, seawater, and groundwater, ranging in size from 1 nm to 1 μm, with a maximum of 108 particles per liter (Lead and Wilkinson, 2006; Gibson et al., 2007). They have different sizes, shapes, complex components, huge specific surface area, and strong adsorption capacity (Buffle, 2006; Hochella et al., 2008; Auffan et al., 2009). Colloid is an important carrier of nutrient organic carbon, nutrients, trace metals, trace organic matter, pollutants, and pigments. The colloid is considered the "bridge" between the true solution phase and the particle phase, acting as a "colloid pump" (Dai and Benitez-Nelson, 2001). At present, the colloid research of natural water bodies focuses on water bodies such as oceans, bays, lagoons, rivers, and estuaries (Orlandini et al., 1990; Guo et al., 1994; Ran et al., 2000; Dai and Benitez-Nelson, 2001; Stolpe et al., 2010). In recent years, the role of natural colloids in lake biogeochemical cycles has also attracted attention (Shirokova et al., 2013). Shallow lakes refer to those whose water depth is less than 5 m and without obvious water temperature stratification in summer (Nixdorf and Deneke, 1997). Compared with deep-water lakes and oceans, shallow-water lakes have a larger water-soil contact area per unit volume, and the disturbance of bottom mud by hydrodynamic effects such as wind and waves is more frequent and obvious (Xu et al., 2018b). As the third largest freshwater lake in China, Taihu Lake is a typical large shallow lake. For large shallow lakes, frequent sediment resuspension and cyanobacteria blooms, as well as complex exogenous and diverse ecosystem structures, make the source, material composition, and environmental behavior of colloids in shallow lakes more complicated. Therefore, it is important to study the release, migration, and transformation of lake sedimentary colloids.
At present, colloids in shallow lakes focused mostly on the colloidal organic carbon, metal elements, and nutrient content (Moran, 1991; Dai et al., 1995; Zhang and Wang, 2004; Shirokova et al., 2013). In order to better understand how sedimentary colloids affect their surrounding environment and the behavior and fate of sedimentary colloids under different environmental conditions (pH, cations, and the existence of organic matter), it is necessary to evaluate their stability and aggregation research (Tombácz et al., 2001; Heidmann et al., 2005; Lee et al., 2011; Liu et al., 2018). The GMZ (Inner Mongolia Gaomiaozi region, China) bentonite colloids were more stable at low cation concentrations and high pH conditions, when salinity or acidity increases, the aggregation become obvious (Xu et al., 2018a, 2020). Humic substances exert a strong influence on colloid properties even at low salinity (Yang et al., 2019). Biochar colloids from lower pyrolysis temperature and smaller particle size have stronger fluidity in porous media (Wang et al., 2013). Above the point of zero charge (PZC) of the soil colloid, the soil colloid tends to be dispersed. When environmental pH is close to its PZC, the soil colloid tends to aggregate (Kretzschmar et al., 1998; Saxena et al., 2019). At the same time, the size of the molecular weight will also affect the agglomeration behavior of the electrolyte on the sedimentary colloid (Xu et al., 2016b). At present, the main techniques for colloid separation are sedimentation, tangential ultrafiltration, and size exclusion chromatography (Artinger et al., 2000; Morrison and Benoit, 2004; Belzile and Guo, 2006), etc. Molecular characterization of colloids has therefore become a primary research objective in environmental and ecological chemistry (Nebbioso and Piccolo, 2011). Studies have shown that up to 76% of the total organic carbon in the aquatic environment is colloidal organic carbon (COC) (Dai and Benitez-Nelson, 2001). The stability of nanoparticles will be greatly affected by the aromaticity of organics and the type of functional groups (Hyung et al., 2007; Louie et al., 2013). For example, organic aromatic components in the environment could inhibit the aggregation behavior of carbon nanomaterials (Hyung et al., 2007). However, because of the great complexity and heterogeneous composition of colloids, its characterization remains a challenge (Nebbioso and Piccolo, 2013). The aggregation behavior of sedimentary colloids from different sources and different particle sizes and the role of electrolytes need to be further studied.
The purpose of this article is to: 1) characterize sedimentary colloids in two different regions and determine the source of the colloids; 2) evaluate the stability of sedimentary colloids through different environmental factors, and electrolyte types and concentrations. To achieve these goals, sedimentary colloids were extracted from macrophyte-dominated area (MD) and algae-dominant area (AD) in this study. The critical coagulation concentration (CCC) of sedimentary colloids in different electrolytes (NaCl, CaCl2, and MgCl2) was also measured to evaluate the stability of the particles.
2 MATERIAL AND METHOD 2.1 Collection of lake sediment samplesSampling was taken from macrophyte-dominant area (MD) and algae-dominant area (AD) of Taihu Lake (30°55'40"N–31°32'58"N; 119°52'32"E–120°36'10"E) in Jiangsu Province. The samples were collected in June 2020 (see Supplementary Table S1 for details). After collection, the sediment samples were transported to the laboratory and stored at 4 ℃ in the refrigerator for use.
2.2 Extraction and classification of sedimentary colloidal particlesWeigh 50 g of sedimentary from MD and AD in a 500-mL beaker, add ultrapure water to adjust the sediment to water ratio to be 1∶10, and store at 4 ℃ for 24 h. The suspend was shaken under 25 ℃ for 6 h at 200 r/min (Yasutaka et al., 2017). After shaking, the suspends were centrifuged at 3 000 r/min for 20 min. Then, the supernatant was filtered through 1-μm membranes and then the obtained macrophyte-dominant area colloids (MDCs) and algae-dominant area colloids (ADCs) were passed through 0.45-μm membranes, and the filtered leachate was analyzed. At last, the solution was cut into colloids with different molecular weights by cross-flow ultrafiltration (CFUF). In the end, the colloids with four molecular weight ranges from two different regions were obtained, including MDCs-A–D and ADCs-A–D. (A: 0.45–1 μm; B: 1 000 kDa–0.45 μm; C: 100–1 000 kDa; D: 10–100 kDa (Cheng et al., 2018). The colloidal solutions of different molecular weights were freeze-dried for the following study.
2.3 Sedimentary colloids condensation experimentIn this study, pH, temperature, and ionic strength-dependent aggregations of sedimentary colloids measured over a wide range of pH (4–9), temperature (0–35 ℃), and ionic strength (Ca2+, Mg2+ and Na+). The dynamic light scattering technology was used to monitor the aggregation kinetics process of four colloids with different particle sizes. The hydraulic diameter of the sedimentary colloids was measured every 15 s for a total of 1 800 s. Zeta potential of colloids was measured using a Malvern Zetasizer Nano ZS equipped with a 633-nm wavelength laser beam and a detection angle of 173°. The electrophoretic mobility was converted to zeta potential using an algorithm based on the Smoluchowski theory. All colloidal samples were sonicated in an ultrasonic bath for 10 min immediately before measurement.
2.4 Evaluation of sedimentary colloids stabilityThe critical coagulation concentration (CCC) was used to estimate the stability of nanoparticles in electrolyte solution (Chen et al., 2006). And the attachment efficiency (a) was introduced to obtain the CCC value accurately.
The equation below depicts the dependence of the initial aggregation rate constant (k) upon initial rate of linear increase and initial colloids concentration (N):
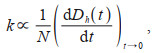
where Dh(t) is the hydraulic diameter of colloid particles at t moment.
The aggregation attachment efficiency (a, equivalent to the inverse stability ratio) ranging from 0 to 1 was used to quantify the aggregation kinetics under different solution conditions. a is calculated by normalizing the initial slope of aggregation under different solution conditions to the slope obtained under diffusion-limited (fast) aggregation conditions:
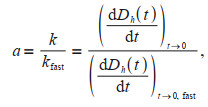
where k is the aggregation rate constant for various solutions, kfast is the aggregation rate.
2.5 Analyses methodThe Zetasizer Nano (Malvern) was used to measure the ζ potential of the sedimentary colloid particles. The UV-visible absorption spectroscopy was performed on a spectrophotometer (Varian Cary 5000) over a wavelength range of 200–800 nm with 1-nm increments. All aqueous samples were analyzed for organic carbon with a total organic carbon (TOC) analyzer (Shimadzu TOC-VCPH/CPN). Fluorescence excitation-emission matrix (EEM) spectra were measured using fluorescence spectrophotometer (Horiba, FM-4P-TCSPC) (Benner et al., 1993). The spectra were collected by changing the excitation wavelength from 200 to 450 nm at a 5-nm increment, with subsequent scanning emission spectra from 250 to 700 nm. The spectra were recorded at scanning speed of 1 200 nm/min.
3 RESULT AND DISCUSSION 3.1 Characterization of sedimentary colloids 3.1.1 The morphology and size of sedimentary colloidsThe sedimentary colloids of different particle sizes from different ecological regions are shown in Fig. 1. No significant difference in the shape was observed between the MDCs and ADCs. Both colloids are contained a mixture of polygonal, spherical, and elliptical shape, and their sizes ranged 200–500 nm in uneven size distribution. Colloids may cluster or form aggregates in aqueous suspensions, although TEM measurement itself could result in some artifacts (Li et al., 2012). The size of the particles was less than 1 μm, and even some of the particle size reached the nanoscale. In addition, some organic-coated boundary can be found on the surface of sedimentary colloids, which would be attributed to the chemical and/or physical sorption of macromolecules.
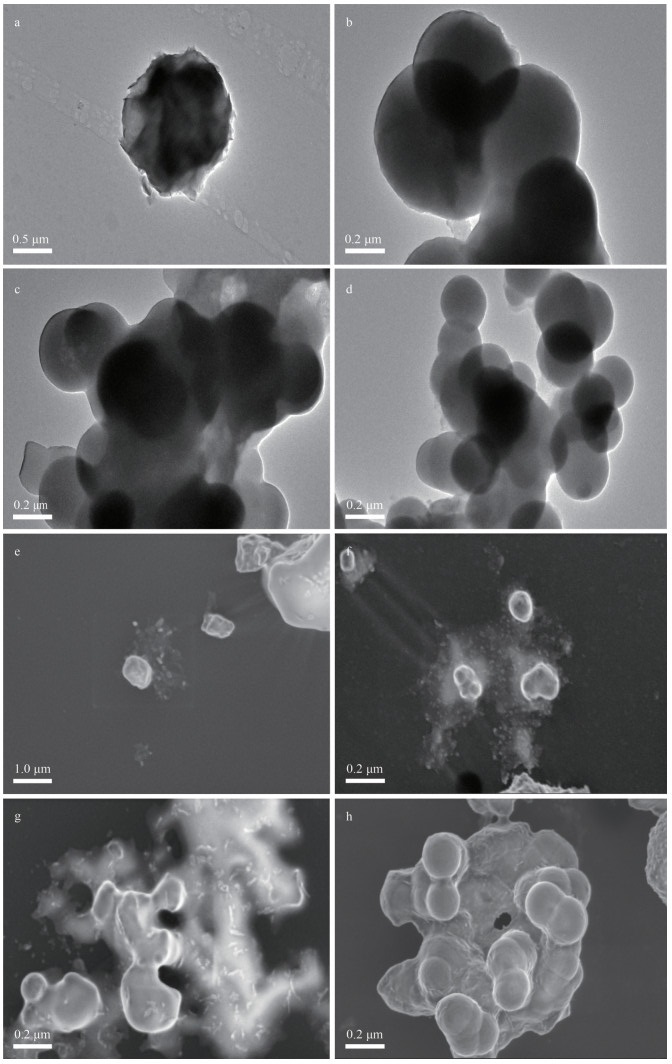
|
| Fig.1 TEM of the sedimentary colloids from two different regions a–d. macrophyte-dominant area colloids-A–D (MDCs-A–D); e–h. algae-dominant area colloids-A–D (ADCs-A–D). |
The UV-vis spectra of the colloids at 200–800 nm are shown in Supplementary Fig.S1. Further analysis showed that the absorbance decreased with the decreasing molecular weight in both MDCs and ADCs.
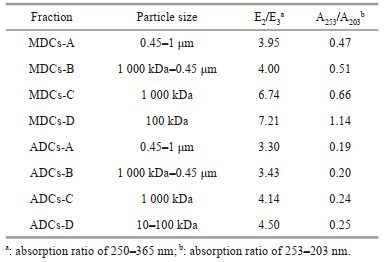
The characteristics of MDCs and ADCs can also be expressed by the absorption ratio, which is defined as the ratio of the absorption coefficients of two different wavelengths, and is often used to reflect the molecular weight, aromaticity, and degree of humification of sedimentary colloids (Li and Hur, 2017). For example, E2/E3 (250/365 nm) is used to characterize the molecular weight of the colloids, and it is negatively correlated with the molecular weight (Duarte et al., 2005; Erlandsson et al., 2012; Santos et al., 2016). It can be seen in Table 1, with the decrease of molecular weight of colloids, E2/E3 value increase from 3.95 to 7.21 and from 3.30 to 4.50 in MD and AD area, respectively. Especially, the E2/E3 ratios of the colloids are all greater than 3.5, which indicates that the organic matter of the sedimentary colloid is mainly fulvic acid, and as the molecular weight decreases, the proportion of fulvic acid gradually increases (Teng et al., 2020; Zhang et al., 2020).
|
In addition, A253/A203 was used to measure the aromaticity of MDCs and ADCs (Korshin et al., 1997), the aromaticity of the colloid increase with the A253/A203 value. In general, the A253/A203 value of MDCs is significantly higher than ADCs (Table 1), which is nearly 2–5 times, indicating that MDCs contains a higher degree of aromatization, and the substituent of ADCs are mainly aliphatic chains (Rodríguez et al., 2016).
3.1.3 Fourier transform infrared spectroscopy (FT-IR) analysis of sedimentary colloidsIn order to characterize the compositions of the sedimentary colloids, FT-IR techniques were applied. Figure 2 show some obvious absorption peaks, e.g., 3 460, 1 650, 1 020 cm-1, can be observed from MDCs and ADCs. The peaks at 3 460 cm-1 were attributed to the internal -OH groups of kaolin material; peaks at 1 650 cm-1 attributed to C=C stretching vibration of the aromatic group; peaks at 1 020 cm-1 wereattributed to asymmetric stretching vibration of C-O. Further analysis showed that the absorption peak of LMW-colloids deposited in the two regions was stronger than HMW-colloid at 1 450–1 400 cm-1, indicating that the small molecular weight part contains more oxygen-containing functional groups. At 1 620–1 650 cm-1, LMW-colloids have more aromatic ring structure and aliphatic chain structure. And HMW-colloids had a more obvious absorption at 1 050–1 200 cm-1, indicating that it more polysaccharides (Leenheer, 1981; Niemeyer et al., 1992; Chefetz et al., 1998; Smidt and Meissl, 2007).
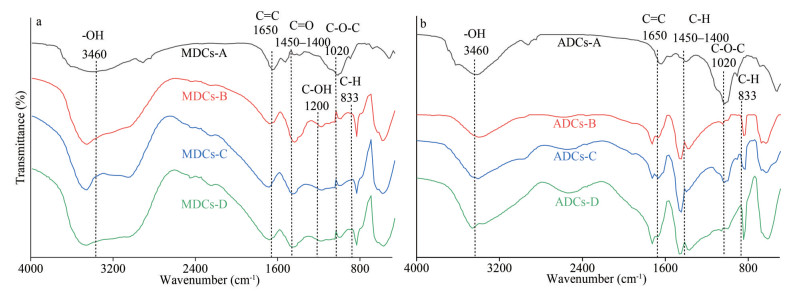
|
| Fig.2 FT-IR patterns of sedimentary colloids and its fractions a. MDCs; b. ADCs. |
In short, LMW-colloids contain more oxygen-containing functional groups and aromatic structures, while HMW-colloids contain more carbohydrates. However, the composition of sedimentary colloids in different ecology environment is similar, but there are differences in content. The presence of hydroxyl and carboxyl groups could affect the agglomeration of ions on colloids.
3.1.4 Three-dimensional fluorescence spectra of sedimentary colloidsThe sedimentary colloids consist of a variety of fluorescent structure, their fluorescence spectra contain abundant and interwoven fluorescence information. As shown in Fig. 3, compared with ADCs that has peak A only (humic-like structure with two or more aromatic rings, 320–380/430–460 nm), there are three typically pairs of fluorescence peaks in MDCs (Coble et al., 2014; Baker et al., 2015; Carstea et al., 2016; Fox et al., 2017): peak A, peak B (fulvic-like structure, 250–275/450–490 nm), and peak C (protein-like 270–290/315–365 nm). The appearance of peak A that exists in mainly LWM-colloids, was believed to be related to the conjugation effect caused by the carboxyl and carbonyl groups in the structure (Yan et al., 2013; Xu et al., 2016a). This result is consistent with the previous IR results. At the same time, peak B is contributed by aromatic phenols and aliphatic substances (Chen et al., 2003; Gu et al., 2018). In general, the result indicates humic-like substances exist in LWM-colloids mainly, and humic-like substances decreased gradually with the increasing colloids molecular weight. Similar results have also been observed in the molecular weight-fractionation of natural organic matter in Suwannee River (Shen et al., 2015). Comparing different ecology regions, MDCs contained more humic acids and protein-like substances than ADCs.
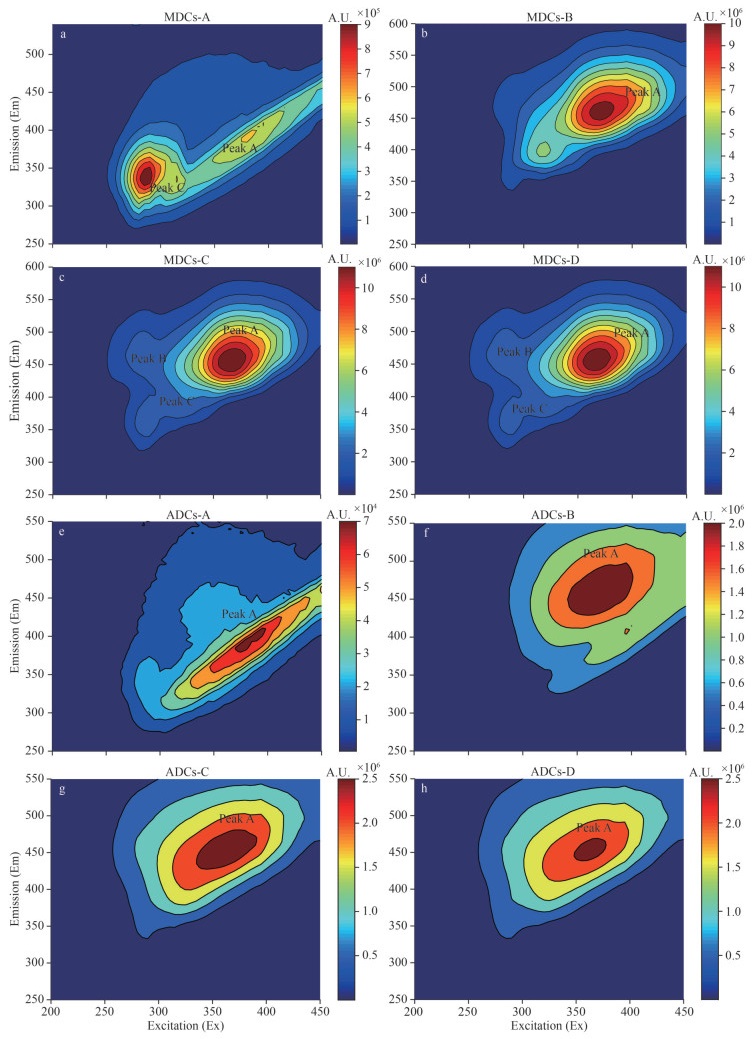
|
| Fig.3 Three-dimensional fluorescence spectra of different molecular weight a–d. MDCs-A–D; e–h. ADCs-A–D. |
The zeta potential (electro-kinetic potential) is the potential on the sliding surface of the colloidal electric double layer, which is closely related to the charge on the surface of the colloidal particles. When surface of the colloidal particles is positively charged, the surface potential is positive. On the contrary, if the surface of the colloidal particles is negatively charged, the surface potential is negative. In short, the zeta potential will have a significant impact on the stability of the entire system. When the absolute value of zeta potential on particles in the solution is higher than 30 mV, the dispersion system should be relatively stable (Gibson et al., 2009).
It can be seen from Fig. 4, the zeta potentials of sedimentary colloids in different ecology environments are all negative, and the absolute value is greater than 30 mV. The electro-negativity of MDCs is obviously greater than that of ADCs, indicating that the stability of MDCs is better than ADCs. With the decrease of colloids molecular weight, the zeta potential value increased from -30.1 to -40.7 mV and from -29.8 to -32.1 mV in MD and AD areas, respectively, it can be found the increasing zeta potential value of MDCs (about 10 mV) is greater than that of ADCs (about 2 mV). As the result of dissolution/dispersion can resist aggregation, the colloids become more stable when molecular weight decrease.
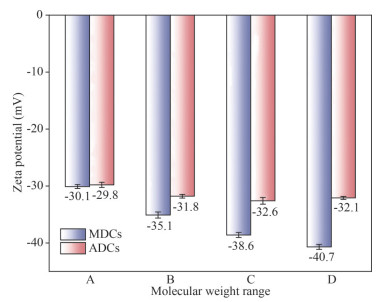
|
| Fig.4 Zeta potential of different molecular weight MDCs and ADCs A: 0.45–1 μm; B: 1 000 kDa–0.45 μm; C: 100–1 000 kDa; D: 10–100 kDa. |
With the increase of temperature, the zeta potential values of all colloids showed an upward trend, indicating that the stability of colloids has decreased (Fig.5a–b). Take HMW-colloids for example, the zeta potential of MDCs and ADCs both increased more than 10 mV. As the temperature rises, the Brownian motion of the colloids accelerates, and the collisions between each other become more frequent. The charges on the colloidal surface transfer to each other during the collision and become unstable, which causes the zeta potential of the colloid to decrease. Meanwhile the aggregation time was shortened and the colloidal stability was reduced. In addition, the zeta potential of the colloids changes greatly below 15 ℃, especially, the variation range of ADCs is relatively greater than that of MDCs. When temperature is above 15 ℃, the growth trend becomes gentler; from the perspective of molecular weight, the LMW-colloids are more stable than HMW-colloids.
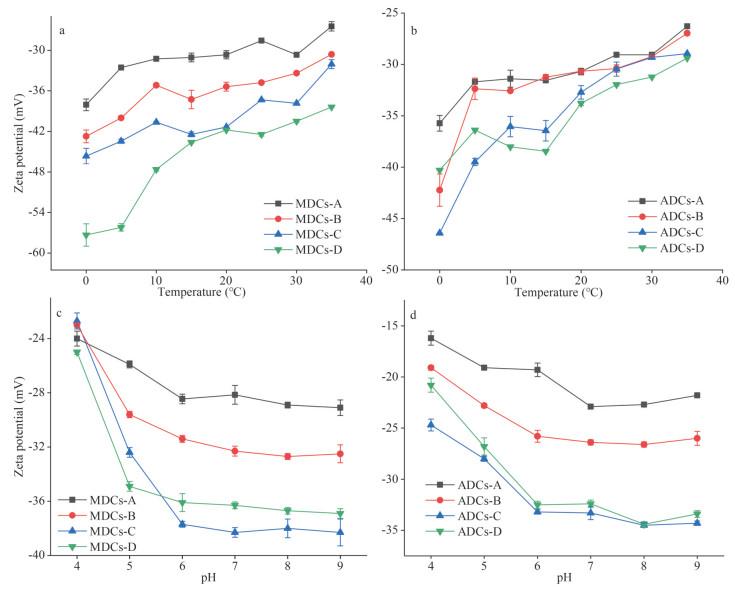
|
| Fig.5 Trend of zeta potential of MDCs and ADCs at different temperature (a–b) and pH (c–d) |
The changing trends of colloids under different pH values are quite different. In pH range of 4–6, the negative potential of zeta drops rapidly, and the zeta potential of MDCs drops more than 10 mV. However, in the pH range of 7–9, the zeta potential decreases slowly, and the zeta potential of MDCs drop less than 1 mV. The Structure of colloids is very sensitive to ambient pH conditions because it contains various functional groups susceptive to protons (Chen et al., 2019). The result shows that the surface of the colloid is a variable charge surface with exposed positive and negative charges. Under alkaline conditions, OH- can neutralize the positive charge on the surface of the colloid, causing more negative potential points to be released on the surface of the colloid, resulting in a drop-in zeta potential; at the same time, Na+ is also diffused and distributed near the surface of the colloid due to electrostatic adsorption. Under acidic conditions, the negative potential point on the surface of the variable charge disappears; H+ in the solution has different electron double layers due to different components. When the concentration of H+ is low, H+ would manifest the effect of base ions compressing the electric double layer, which induces slow condensation of colloids. With the increase of H+ concentration, the surface potential is rapidly reduced by surface neutralization, which causes rapid sedimentation. Further analysis reveal MDCs are more stable than ADCs under neutral and alkaline conditions.
3.2.2 Aggregation of sedimentary colloids in electrolyte solutionsIn this study, aggregation and attachment efficiency of different-molecular-weighted colloids from different ecology environments were calculated based on the hydrodynamic diameter data (Fig. 6). The aggregation adhesion efficiency (a) was used to quantitatively describe the aggregation behavior of colloids under the action of different electrolytes (Hu et al., 2010). Critical coagulation concentration (CCC) is obtained by the intersection of the interpolated line of fast aggregation and slow aggregation, and it represents the highest electrolyte concentration required to maintain the stability of the colloid (Chen and Elimelech, 2006; Saleh et al., 2008; Bouchard et al., 2012). CCC can clearly analyze the influence of different electrolytes, the properties of the colloid itself, and the influence of different molecular weights on the stability of the colloid. It can be seen from Fig. 6 that when the electrolyte solution (Na+, Ca2+, Mg2+) is gradually added to the colloids, the aggregation and adhesion efficiency of the colloid increases rapidly. Finally, its aggregation efficiency tends to stabilize, and fluctuating around 1.
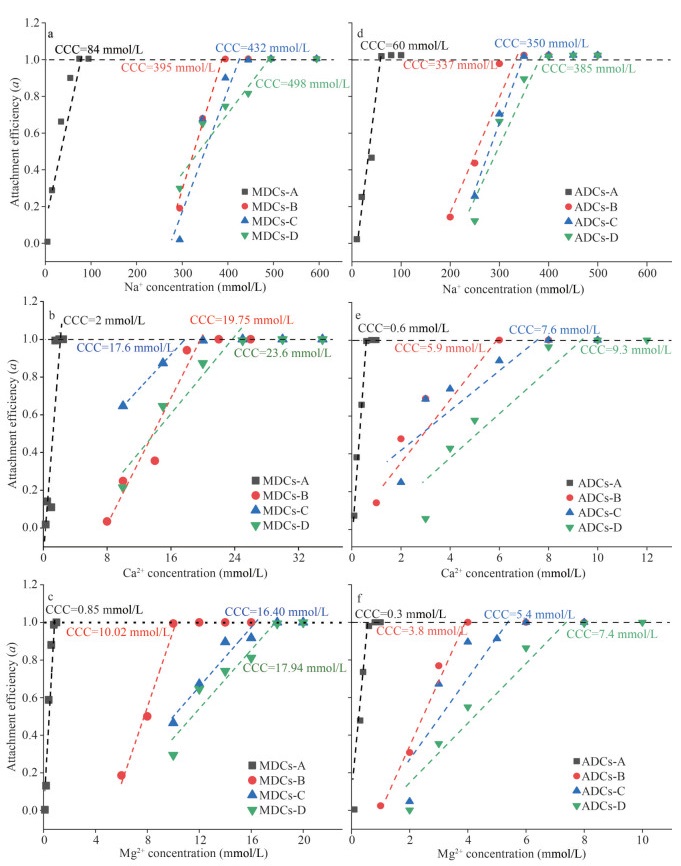
|
| Fig.6 Electrolyte-dependent variations in attachment efficiencies of sedimentary colloids and its fractions a–c. MDCs; d–f. ADCs. |
It was shown that the CCC values were highly dependent on the valence of electrolytes. Take HMW-colloids for example, the CCCs in Na+-treatment were 84 and 60 mmol/L for colloids from the MD and AD regions, respectively. However, in Ca+-treatment, CCCs decreased to 2 and 0.6 mmol/L; in Mg2+-treatment, CCCs decreased to 0.85 and 0.3 mmol/L, respectively. The lower CCC value of Ca2+- or Mg2+-treatment can attribute to higher charge density and stronger neutralization abilities of divalent electrolytes, the bivalent cation can better neutralize the negative charge of the colloid and reduce the stability of the colloid, which is consistent with the previous research results (Stankus et al., 2011; Tan et al., 2019).
Meanwhile, the origin of colloids also played an important role in the CCC values. For instance, the colloids origin from MD regions exhibited higher CCC values than those in AD regions. Specifically, the CCC values of Na+, Ca2+, and Mg2+ were 84, 2, and 0.85 mmol/L for LMW-MDCs, respectively, which were obviously higher than those in AD region (CCCNa+=60 mmol/L, CCCCa2+=0.6 mmol/L, and CCCMg2+=0.3 mmol/L). Based on these CCCs, it is clear that colloids from MD regions were more stable than ADCs in Na+, Ca2+, and Mg2+ electrolyte solutions. The zeta-potentials of MDCs were range from -30.1 to -40.7 mV, while those of MDCs in the range from -29.8 to -32.6 mV. Lower zeta-potentials suggest that more electrolyte cations are needed for colloidal aggregation, which is responsible for the higher CCC values of Na+, Ca2+, and Mg2+ of MDCs.
In addition, the molecular weights of colloids played an important role in determining the CCC values. Overall, HMW-colloids exhibited higher CCC values than LMW-colloids both for the macrophyte- and algae-dominant sediments. For example, in MD areas, with the decrease of colloidal MW, CCCs values increase from 84 (MDCs-A) to 395 (MDCs-B), 432 (MDCs-C), and 498 (MDCs-D) mmol/L for Na+. The results clearly demonstrate that the LMW-colloidal stability was more efficient than the HMW-colloidal. The heterogeneous aggregation of colloids is mainly due to the fraction of organic matrix. Combining the analysis of previous three-dimensional fluorescence and infrared images, it can be seen that the content of humic acid in LMW-colloids is much higher than that of HMW-colloids. The presence of humic acid is an important factor affecting the stability of the colloid. Therefore, the influence of electrostatic force on the stability of sediment colloids is greater than that of the van der Waals force, which will lead to more stable colloids. The electrostatic effect (electrostatic repulsion) associated with organic matter plays an important role in the change of colloidal stability (Mashayekhi et al., 2012).
3.3 Significance and implicationEutrophic shallow lakes, such as Taihu Lake, usually have the characteristics of high abundance of MDCs and ADCs. Previous studies focused on the characterization and origin of colloids. However, the influence of different molecular weight components on colloidal stability was neglected. In this study, the importance of molecular weight distribution on colloidal stability is emphasized, which indicate that heterogeneity of molecular weight distribution should be considered when explaining the influence of organic matters on colloidal stability and predicting the behavior colloidal.
Furthermore, since sedimentary colloids is highly heterogeneous in molecular weight and composition, different MW-colloids are expected to play distinct roles in determining the aggregation behavior. We found evident MW-dependent aggregation heterogeneity in both mono- and divalent electrolytes with the 10–100-kDa LMW-colloids having higher stability efficiency. The results highlight the heterogeneity of colloids in regulating colloidal stability among different MW fractions within sedimentary colloids.
Finally, at the concentration related to the environment, i.e., Na+, Ca2+, and Mg2+; Ca2+ and Mg2+ can more effectively enhance the colloidal stability than Na+. As reported, fresh waters usually contain Na+, Ca2+, and Mg2+, this also accounted for the persistence of turbidity in many aquatic ecosystems.
4 CONCLUSIONEffects of MW fractions and chemical properties of sedimentary colloids in different ecology environment from Taihu Lake were explored. There are significant differences in the molecular weight and content of organic matter in the MDCs and ADCs; MDCs are mainly composed of humic acid, protein, and fulvic acid, while ADCs are mainly composed of humic acid. The presence of humic acid in colloids improved the electrostatic repulsion between colloidal particles, which could effectively inhibit the agglomeration of colloids, thereby enhancing the colloid stability. LMW-colloids and colloids in MD area show better stability. Furthermore, the increase of temperature reduces the stability of the colloids, and the stability of colloids increases with the increasing pH under neutral or alkaline conditions. Both monovalent and divalent electrolytes can promote colloid agglomeration. It can be found that under the action of the electrolyte, the CCC value of the colloid in any ecology regions increases as the molecular weight decreases, indicating that the stability of the deposited colloid is also increasing. In order to clarify the migration, transformation, and fate of colloidal particles, it is necessary to study the molecular weight distribution characteristics of colloids in detail.
5 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Supplementary material (Supplementary Table S1 and Fig. S1) is available in the online version of this article at https://doi.org/10.1007/s00343-022-2254-3.
Artinger R, Buckau G, Geyer S, et al. 2000. Characterization of groundwater humic substances: influence of sedimentary organic carbon. Applied Geochemistry, 15(1): 97-116.
|
Auffan M, Rose J, Bottero J Y, et al. 2009. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nature Nanotechnology, 4(10): 634-641.
|
Baker A, Cumberland S A, Bradley C, et al. 2015. To what extent can portable fluorescence spectroscopy be used in the real-time assessment of microbial water quality?. Science of the Total Environment, 532: 14-19.
|
Belzile C, Guo L D. 2006. Optical properties of low molecular weight and colloidal organic matter: application of the ultrafiltration permeation model to DOM absorption and fluorescence. Marine Chemistry, 98(2-4): 183-196.
|
Benner R, Hedges J I. 1993. A test of the accuracy of freshwater DOC measurements by high-temperature catalytic oxidation and UV-promoted persulfate oxidation. Marine Chemistry, 41(1-3): 161-165.
|
Bouchard D, Zhang W, Powell T, et al. 2012. Aggregation kinetics and transport of single-walled carbon nanotubes at low surfactant concentrations. Environmental Science and Technology, 46(8): 4458-4465.
|
Buffle J. 2006. The key role of environmental colloids/ nanoparticles for the sustainability of life. Environmental Chemistry, 3(3): 155-158.
|
Carstea E M, Bridgeman J, Baker A, et al. 2016. Fluorescence spectroscopy for wastewater monitoring: a review. Water Research, 95: 205-219.
|
Chefetz B, Hader Y, Chen Y. 1998. Dissolved organic carbon fractions formed during composting of municipal solid waste: properties and significance. Acta Hydrochimica et Hydrobiologica, 26(3): 172-179.
|
Chen K L, Elimelech M. 2006. Aggregation and deposition kinetics of fullerene (C60) nanoparticles. Langmuir, 22(26): 10994-11001.
|
Chen K L, Mylon S E, Elimelech M. 2006. Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes. Environmental Science & Technology, 40(5): 1516-1523.
|
Chen W, Teng C Y, Qian C, et al. 2019. Characterizing properties and environmental behaviors of dissolved organic matter using two-dimensional correlation spectroscopic analysis. Environmental Science & Technology, 53(9): 4683-4694.
|
Chen W, Westerhoff P, Leenheer J A, et al. 2003. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environmental Science and Technology, 37(24): 5701-5710.
|
Cheng D M, Liu X H, Li J P, et al. 2018. Effects of the natural colloidal particles from one freshwater lake on the photochemistry reaction kinetics of ofloxacin and enrofloxacin. Environmental Pollution, 241: 692-700.
|
Coble P G, Spencer R G M, Baker A, Reynolds D M, et al. 2014. 3-Aquatic Organic Matter Fluorescence. Cambridge University Press, Cambridge. p.75--122.
|
Dai M H, Benitez-Nelson C R. 2001. Colloidal organic carbon and 234Th in the Gulf of Maine. Marine Chemistry, 74(2-3): 181-196.
|
Dai M H, Martin J M, Cauwet G. 1995. The significant role of colloids in the transport and transformation of organic carbon and associated trace metals (Cd, Cu and Ni) in the Rh?ne delta (France). Marine Chemistry, 51(2): 159-175.
|
Duarte R M B O, Pio C A, Duarte A C. 2005. Spectroscopic study of the water-soluble organic matter isolated from atmospheric aerosols collected under different atmospheric conditions. Analytica Chimica Acta, 530(1): 7-14.
|
Erlandsson M, Futter M N, Kothawala D N, et al. 2012. Variability in spectral absorbance metrics across boreal lake waters. Journal of Environmental Monitoring, 14(10): 2643-2652.
|
Fox B G, Thorn R M S, Anesio A M, et al. 2017. The in situ bacterial production of fluorescent organic matter; an investigation at a species level. Water Research, 125: 350-359.
|
Gibson C T, Turner I J, Roberts C J, et al. 2007. Quantifying the dimensions of nanoscale organic surface layers in natural waters. Environmental Science and Technology, 41(4): 1339-1344.
|
Gibson N, Shenderova O, Luo T J, et al. 2009. Colloidal stability of modified nanodiamond particles. Diamond and Related Materials, 18(4): 620-626.
|
Gu Z P, Chen W M, Li Q B, et al. 2018. Degradation of recalcitrant organics in landfill concentrated leachate by a microwave-activated peroxydisulfate process. RSC Advances, 8(57): 32461-32469.
|
Guo L D, Coleman C H Jr, Santschi P H. 1994. The distribution of colloidal and dissolved organic carbon in the Gulf of Mexico. Marine Chemistry, 45(1-2): 105-119.
|
Heidmann I, Christl I, Kretzschmar R. 2005. Aggregation kinetics of kaolinite-fulvic acid colloids as affected by the sorption of Cu and Pb. Environmental Science and Technology, 39(3): 807-813.
|
Hochella M F, Lower S K, Maurice P A, et al. 2008. Nanominerals, mineral nanoparticles, and Earth systems. Science, 319(5870): 1631-1635.
|
Hu J D, Zevi Y, Kou X M, et al. 2010. Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions. Science of the Total Environment, 408(16): 3477-3489.
|
Hyung H, Fortner J D, Hughes J B, et al. 2007. Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environmental Science and Technology, 41(1): 179-184.
|
Korshin G V, Li C W, Benjamin M M. 1997. Monitoring the properties of natural organic matter through UV spectroscopy: a consistent theory. Water Research, 31(7): 1787-1795.
|
Kretzschmar R, Holthoff H, Sticher H. 1998. Influence of pH and humic acid on coagulation kinetics of kaolinite: a dynamic light scattering study. Journal of Colloid and Interface Science, 202(1): 95-103.
|
Lead J R, Wilkinson K J. 2006. Aquatic colloids and nanoparticles: current knowledge and future trends. Environmental Chemistry, 3(3): 159-171.
|
Lee M H, Jung E C, Song K, et al. 2011. The influence of humic acid on the pH-dependent sorption of americium (Ⅲ) onto kaolinite. Journal of Radioanalytical and Nuclear Chemistry, 287(2): 639-645.
|
Leenheer J A. 1981. Comprehensive approach to preparative isolation and fractionation of dissolved organic carbon from natural waters and wastewaters. Environmental Science and Technology, 15(5): 578-587.
|
Li P H, Hur J. 2017. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: a review. Critical Reviews in Environmental Science and Technology, 47(3): 131-154.
|
Li W, He Y, Wu J, et al. 2012. Extraction and characterization of natural soil nanoparticles from Chinese soils. European Journal of Soil Science, 63(5): 754-761.
|
Liu Y X, Alessi D S, Flynn S L, et al. 2018. Acid-base properties of kaolinite, montmorillonite and illite at marine ionic strength. Chemical Geology, 483: 191-200.
|
Louie S M, Tilton R D, Lowry G V. 2013. Effects of molecular weight distribution and chemical properties of natural organic matter on gold nanoparticle aggregation. Environmental Science & Technology, 47(9): 4245-4254.
|
Mashayekhi H, Ghosh S, Du P, et al. 2012. Effect of natural organic matter on aggregation behavior of C60 fullerene in water. Journal of Colloid and Interface Science, 374(1): 111-117.
|
Moran S B. 1991. The application of cross-flow filtration to the collection of colloids and their associated metals in seawater. In: Hurd D C, Spencer D W eds. Marine Particles: Analysis and Characterization, Geophysical Monograph Series. American Geophysical Union. 63: 275-280, https://doi.org/10.1029/GM063p0275.
|
Morrison M A, Benoit G. 2004. Investigation of conventional membrane and tangential flow ultrafiltration artifacts and their application to the characterization of freshwater colloids. Environmental Science and Technology, 38(24): 6817-6823.
|
Nebbioso A, Piccolo A. 2011. Basis of a humeomics science: chemical fractionation and molecular characterization of humic biosuprastructures. Biomacromolecules, 12(4): 1187-1199.
|
Nebbioso A, Piccolo A. 2013. Molecular characterization of dissolved organic matter (DOM): a critical review. Analytical and Bioanalytical Chemistry, 405(1): 109-124.
|
Niemeyer J, Chen Y, Bollag J M. 1992. Characterization of humic acids, composts, and peat by diffuse reflectance Fourier-transform infrared spectroscopy. Soil Science Society of America Journal, 56(1): 135-140.
|
Nixdorf B, Deneke R. 1997. Why 'very shallow' lakes are more successful opposing reduced nutrient loads. Hydrobiologia, 342: 269-284.
|
Orlandini K A, Penrose W R, Harvey B R, et al. 1990. Colloidal behavior of actinides in an oligotrophic lake. Environmental Science and Technology, 24(5): 706-712.
|
Ran Y, Fu J M, Sheng G Y, et al. 2000. Fractionation and composition of colloidal and suspended particulate materials in rivers. Chemosphere, 41(1-2): 33-43.
|
Rodríguez F J, Schlenger P, García-Valverde M. 2016. Monitoring changes in the structure and properties of humic substances following ozonation using UV-Vis, FTIR and 1H NMR techniques. Science of the Total Environment, 541: 623-637.
|
Saleh N B, Pfefferle L D, Elimelech M. 2008. Aggregation kinetics of multiwalled carbon nanotubes in aquatic systems: measurements and environmental implications. Environmental Science and Technology, 42(21): 7963-7969.
|
Santos L, Pinto A, Filipe O, et al. 2016. Insights on the optical properties of estuarine DOM-hydrological and biological influences. PLoS One, 11(5): e0154519.
|
Saxena K, Brighu U, Choudhary A. 2019. Coagulation of humic acid and kaolin at alkaline pH: complex mechanisms and effect of fluctuating organics and turbidity. Journal of Water Process Engineering, 31: 100875.
|
Shen M H, Yin Y G, Booth A, et al. 2015. Effects of molecular weight-dependent physicochemical heterogeneity of natural organic matter on the aggregation of fullerene nanoparticles in mono- and di-valent electrolyte solutions. Water Research, 71: 11-20.
|
Shirokova L S, Pokrovsky O S, Moreva O Y, et al. 2013. Decrease of concentration and colloidal fraction of organic carbon and trace elements in response to the anomalously hot summer 2010 in a humic boreal lake. Science of the Total Environment, 463-464: 78-90.
|
Smidt E, Meissl K. 2007. The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Management, 27(2): 268-276.
|
Stankus D P, Lohse S E, Hutchison J E, et al. 2011. Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environmental Science and Technology, 45(8): 3238-3244.
|
Stolpe B, Guo L D, Shiller A M, et al. 2010. Size and composition of colloidal organic matter and trace elements in the Mississippi River, Pearl River and the northern Gulf of Mexico, as characterized by flow field-flow fractionation. Marine Chemistry, 118(3-4): 119-128.
|
Tan L, Yu Z, Tan X, et al. 2019. Systematic studies on the binding of metal ions in aggregates of humic acid: Aggregation kinetics, spectroscopic analyses and MD simulations. Environmental Pollution, 246: 999-1007.
|
Teng C Y, Zhou K G, Zhang Z, et al. 2020. Elucidating the structural variation of membrane concentrated landfill leachate during Fenton oxidation process using spectroscopic analyses. Environmental Pollution, 256: 113467.
|
Tombácz E, Csanaky C, Illés E. 2001. Polydisperse fractal aggregate formation in clay mineral and iron oxide suspensions, pH and ionic strength dependence. Colloid and Polymer Science, 279(5): 484-492.
|
Wang D J, Zhang W, Hao X Z, et al. 2013. Transport of biochar particles in saturated granular media: effects of pyrolysis temperature and particle size. Environmental Science & Technology, 47(2): 821-828.
|
Xu H C, Lv H, Liu X, et al. 2016a. Electrolyte cations binding with extracellular polymeric substances enhanced Microcystis aggregation: implication for Microcystis bloom formation in eutrophic freshwater lakes. Environmental Science and Technology, 50(17): 9034-9043.
|
Xu H C, Xu M W, Li Y N, et al. 2018a. Characterization, origin and aggregation behavior of colloids in eutrophic shallow lake. Water Research, 142: 176-186.
|
Xu H C, Yang C M, Jiang H L. 2016b. Aggregation kinetics of inorganic colloids in eutrophic shallow lakes: influence of cyanobacterial extracellular polymeric substances and electrolyte cations. Water Research, 106: 344-351.
|
Xu Z, Pan D Q, Sun Y L, et al. 2018b. Stability of GMZ bentonite colloids: aggregation kinetic and reversibility study. Applied Clay Science, 161: 436-443.
|
Xu Z, Sun Y L, Niu Z W, et al. 2020. Kinetic determination of sedimentation for GMZ bentonite colloids in aqueous solution: effect of pH, temperature and electrolyte concentration. Applied Clay Science, 184: 105393.
|
Yan M Q, Fu Q W, Li D C, et al. 2013. Study of the pH influence on the optical properties of dissolved organic matter using fluorescence excitation-emission matrix and parallel factor analysis. Journal of Luminescence, 142: 103-109.
|
Yang W, Bradford S A, Wang Y, et al. 2019. Transport of biochar colloids in saturated porous media in the presence of humic substances or proteins. Environmental Pollution, 246: 855-863.
|
Yasutaka T, Imoto Y, Kurosawa A, et al. 2017. Effects of colloidal particles on the results and reproducibility of batch leaching tests for heavy metal-contaminated soil. Soils and Foundations, 57(5): 861-871.
|
Zhang W C, Wang W X. 2004. Colloidal organic carbon and trace metal (Cd, Fe, and Zn) releases by diatom exudation and copepod grazing. Journal of Experimental Marine Biology and Ecology, 307(1): 17-34.
|
Zhang Z, Teng C Y, Zhou K G, et al. 2020. Degradation characteristics of dissolved organic matter in nanofiltration concentrated landfill leachate during electrocatalytic oxidation. Chemosphere, 255: 127055.
|
 2023, Vol. 41
2023, Vol. 41


