Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Zihao, FU Dejiang, LÜ Shuguo, LIU Zhiyuan
- Interaction between macroalgae and microplastics: Caulerpa lentillifera and Gracilaria tenuistipitata as microplastic bio-elimination vectors
- Journal of Oceanology and Limnology, 41(6): 2249-2261
- http://dx.doi.org/10.1007/s00343-023-2298-z
Article History
- Received Aug. 15, 2022
- accepted in principle Oct. 15, 2022
- accepted for publication Nov. 7, 2022
2 College of Marine Sciences, Hainan University, Haikou 570228, China;
3 Hainan Research Academy of Environmental Sciences, Haikou 571126, China
During the past decade, all kinds of plastics have been produced and used for convenience, and resulting in plastic materials account for 60%–80% of total human waste in terrestrial and aquatic environments (Guzzetti et al., 2018; Akdogan and Guven, 2019). Small plastic particles can be formed from plastic waste due to physical and chemical degradation processes, such as ultraviolet light and microbiological activities (Eriksen et al., 2014). Microplastics (MPs) are typically characterized as plastic particles with aerodynamic dimensions smaller than 5 mm, which are abundant in the environment, especially in oceans and freshwater (Chen et al., 2020a; Xu et al., 2020). Typical MPs found in the aquatic environment include polyethylene (PE), polystyrene (PS), polyamides (PA), and polyethylene terephthalate (PET), which have high production and low biodegradation rates (Wu et al., 2019). Researchers have piqued the interest of MPs due to their pervasiveness and possible ecological damage to the environment.
MPs can be discovered in large quantities in marine environments, posing a major threat to the marine ecosystem (Haward, 2018). However, apart from reducing emissions at the source, little study has been done on how to address the MPs pollution problem. Several technologies, such as reverse osmosis (RO), dissolved air flotation (DAF), sol-gel technique (SGT), dynamic membranes (DM), electrocoagulation, etc., have been used in wastewater treatment plants (WWTPs) to remove MPs from surface water in recent years (Talvitie et al., 2017; Perren et al., 2018; Liu et al., 2021; Yaseen et al., 2022). However, these MPs removal procedures are costly, and the chemical reagents often cause secondary pollution (Zhang and Chen, 2020). Furthermore, the existing methods have only been applied to surface wastewater, which is unsuitable for MPs removal in the ocean. MPs contamination of the marine environment and its adverse ecological effects has recently been recognized as a global concern, but research on how to effectively remove MPs from the seawater is limited (Lebreton et al., 2017; Miller et al., 2020; Gao et al., 2021).
Macroalgae, as the primary producers of marine ecosystems, play an essential role in the biogeochemical circulation and maintaining marine biodiversity (Taylor and Cole, 1994; Chen et al., 2020b). Moreover, the farmed macroalgae contributes to achieve carbon neutralization, mitigating deoxygenation and eutrophication of the water environment (Gao et al., 2022). With growing concern over marine MPs pollution, macroalgae can be loaded with MPs, and are considered a significant temporary reserve for MPs in the aquatic environment (Feng et al., 2020a, b; Gao et al., 2020; Li et al., 2020a; Peller et al., 2021; Sfriso et al., 2021; Zhang et al., 2022). Feng et al. (2020a) discovered that adhesion is the primary mechanism by which MPs are trapped in macroalgae, and the concentrations of MPs on macroalgae were 34–160 times higher than in seawater. Macroalgae can secrete extracellular polymeric substances (EPS) on their surface, which endows macroalgae with a stronger surface viscosity and electrostatic adsorption ability to adsorb MPs (Chi et al., 2018). Greater biomass also makes it easier for macroalgae to trap MPs from the environment, which is attributed to the spatial structure of denser individual compositions (Zhang et al., 2022). Peller et al. (2021) investigated the potential of Cladophora for bioremediation of microfibers in the aquatic environment, and they found that at least half of the microfibers adhered to the surface of seaweeds after 15-h exposure. Therefore, the enrichment properties of macroalgae for MPs can be used to develop phytoremediation strategies to remove MPs from the aquatic environment. It is essential to collect macroalgae from the ocean, otherwise, MPs attached to the algae will enter the food web or be released into the environment with the seaweed dying (Wright et al., 2013; Goss et al., 2018). However, collecting wild seaweeds such as Ulva from the ocean is expensive, and there is little research on the methods of removing MPs from macroalgae. Gutow et al. (2016) found that dewatering and seawater washing reduced the average MPs density on Fucus vesiculosus by 55%–98%. While if a decontamination step is incorporated into the regularly harvested process of farmed macroalgae, commercially clean algae can be produced while reducing the abundance of MPs in seawater. As a result, farmed seaweeds might be an ideal carrier for the bioremediation of MPs. Therefore, Gracilaria tenuistipitata and Caulerpa lentillifera, the two typical tropical farmed seaweed in Hainan Province, China, were explored for MPs biosorption vectors in the present study.
Application of seaweed in phytoremediation is based on studies of the impact of MPs on seaweed. However, a limited number of studies have focused on the effects of MPs on macroalgae, and there is no agreement on current experimental results. Khandare et al. (2022) discovered that PVC degraded products improved growth rate and increased chlorophyll-a and -b contents in Ulva lactuca. Some studies reported that some types of MPs had no effect on the growth of macrophytes (Mateos-Cárdenas et al., 2019; Kalčíková et al., 2020; Rozman et al., 2021). However, the growth of Ulva prolifera in the Yellow Sea during the green tide period was inhibited by high concentrations of polystyrene (PS), and as human food, it may directly threaten human health (Feng et al., 2020a). Therefore, the effect of MPs on macroalgae is complicated and depends on the types of both the MPs and the macroalgae. Polystyrene (PS) is a typical representative in most MPs toxicological studies on the toxicological effects of MPs on algae (Chae et al., 2019; Hazeem et al., 2020; Li et al., 2020b). While polyamide (PA) fibers were mainly composed of MPs in the mariculture area of eastern Hainan (Zhang et al., 2020). So far, no studies have explored the effects of MPs (PA and PS) on macroalgae such as G. tenuistipitata and C. lentillifera.
In this study, to explore one feasible biological approach to reducing the risk of marine MPs in Hainan, the effects of PS particles and PA fibers on two local farmed macroalgae (C. lentillifera and G. tenuistipitata) were investigated, and then the efficient decontamination techniques on the removal of MPs from the algal surfaces were evaluated.
2 MATERIAL AND METHOD 2.1 Seaweeds cultureEntire plants of Caulerpa lentillifera were collected from the shoal area of Fengjia Bay in Hainan Province, China (19.40°N, 110.69°E) on February 26, 2022. Rhodophyta, Gracilaria tenuistipitata, were obtained from the mudflat of Qiuhai Lake in Hainan Province, China (20.05°N, 110.31°E) on March 20, 2022. The seaweeds were transported to the laboratory and cultured for at least one month before being used for the experiment. Seaweeds growth in a recirculating seawater system with aeration at 27±1 ℃, an irradiance of 125 μmol photons/(m2·s) and a 12-h light꞉12-h dark regime. Seawater was collected from Xiuying Pier and filtered through a 0.22-μm filter to remove MPs. The density of algal breeding was 15-g fresh weight per 1-L seawater.
2.2 Effect of MPs on the seaweedAn amount of 5-μm polystyrene (PS) uni-bead particles solution (25 mg/mL, Baseline, Tianjin, China) and 500-μm polyamide (PA) fibers (Baseline, Tianjin, China) were dispersed in the filtered seawater with an ultrasonic bath to achieve the final gradient concentrations of 0, 25, 50, 75, and 100 mg/L. This toxicity test used a high concentration of 100 mg/L as the upper limit of concentration, which can be compared with previous research published by Feng et al. (2020a) and Rozman et al. (2022). The seaweeds were washed with the filtered seawater about three times. Approximately 3 g (fresh weight) of healthy seaweeds were cultured in 250-mL flasks with 200-mL MPs test solutions at concentrations of 0, 25, 50, 75, and 100 mg/L. The growth and physiological indicators of the seaweeds were measured after 7 days of MPs exposure to investigate the response of the two seaweeds to the MPs. All the experimental apparatuses were washed with filtered seawater and sterilized, and each treatment was conducted in triplicate.
2.3 Adsorption and desorption of MPs by seaweedThe microplastic adherence experiment design was based on the method used by Gutow et al. (2016) and has been refined. All experiments were conducted under the controlled conditions (27±1℃, 125 μmol photons/(m2·s), 12-h light꞉12-h dark regime). The branches of C. lentillifera and G. tenuistipitata with a surface area of 0.8–3.1 cm2 were placed in a 50-mL erlenmeyer flask containing 20-mL filtered seawater with a concentration of 977 particles/mL of fluorescent PS microspheres (5 μm, Excitation 488 nm/Emission 518 nm) (Baseline, Tianjin, China) or PA fibers (500 μm, 1 000 items/mL). The flasks were sealed and placed in an orbital shaker at 60 r/min for 72 h. After the seaweeds were carefully removed from the flasks with a tweezer, the number of MPs on the algal surface was counted under the fluorescence microscope or stereo microscope. The surface area of the algal piece was measured by photographs, which were then analyzed using the software ImageJ. The MPs adsorption ability of the seaweeds was expressed by items per unit area (cm2) of the algae.
The decontamination process includes dewatering, washing, or the combined dewatering and washing procedures. For dewatering, the algae were left in the air for 4 h and were tapped 5 or 6 times with a glass rod. For washing, the algae were placed in a Petri dish with 20 mL of filtered seawater free of MPs, and were agitated in the orbital shaker at 100 r/min for 4 h. The number of MPs on the algal surface was counted, and the desorption rate was calculated according to Eq.1:
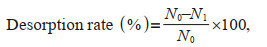 (1)
(1)where N0 is the initial adhesion number of MPs on algal surfaces; N1 is the adhesion number of MPs on the algae surface after decontamination. Each treatment was performed in six replicates.
Two gradient experiments were conducted to investigate the effect of washing factors on microplastic elution. Seaweeds were treated with MPs for 72 h before the experiment (contamination). In the first experiment (Exp.1), the seaweed samples were placed in a Petri dish with 20 mL of filtered seawater free of MPs, and were agitated in the orbital shaker at 100 r/min for 2, 4, 8, 16 h. In the second experiment (Exp.2), the seaweed samples were put into new reagent bottles with filtered seawater in volume gradients of 10, 20, 40, 80, and 160 mL. All bottles were agitated in the orbital shaker for 4 h. The number of MPs on the algal surface was counted under the fluorescence microscope or stereo microscope.
2.4 Parameters analysisThe growth of seaweeds was determined by fresh weight changes weekly. The relative growth rate (RGR) was calculated as follows (Glenn and Doty, 1992):
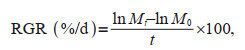 (2)
(2)where M0 is the initial fresh algal biomass; Mt is the fresh mass after t days of cultivation and t is the exposure time (day).
Based on the black and white bottle method (Figueroa et al., 2003), total photosynthetic O2 evolution rates of seaweed were measured by changes in dissolved oxygen concentrations. The oxygen content in the bottle is measured by portable dissolved oxygen meter (ST300D, Aohaosi, America). The total photosynthetic rate was expressed by the rate of photosynthetic oxygen evolution.
Chlorophyll-a concentration was determined according to Wellburn (1994). Malondialdehyde (MDA) content was determined by the thiobarbituric acid method (Pancha et al., 2015). The extracellular polymeric substances (EPS) of seaweeds were extracted using the method proposed by Sfriso et al. (2021), and the content of expolysaccharide was measured using the sulphate-anthrone method (Hassid and Abraham, 1957).
The surface area of the seaweeds was identified by taking photographs with Huygens Essential software and analyzed using ImageJ. The number of fluorescent PS particles on the surface of the algae was manually counted using a fluorescence biological microscope (IX71, Olympus, Japan) with an objective magnification of 40×. The number of PA fibers was counted by Stereo Microscopes (SMZ-161, Motic, China).
2.5 Statistical analysisThe Shapiro-Wilk test and the Levene's test were used to test the normality and homogeneity of variance of the data from each treatment. For data with non-normal distribution and when variance homogeneity was not achieved, the Mann-Whitney U test was performed. In the MPs desorption experiment, using restricted maximum likelihood (REML) and a one-way design with decontamination procedures as a factor, we fitted a variance analysis model, which was then contrasted using Akaike Information Criteria (AIC). The statistical analysis was performed using one-way analysis of variance (ANOVA) in SPSS (Version 26). The Mauchly's test revealed that the assumption of sphericity was satisfied. The difference between the data with a significance level of P < 0.05 was determined using the least significant difference (LSD). Based on parallel experiments, means and standard deviations of the means were used to express the results.
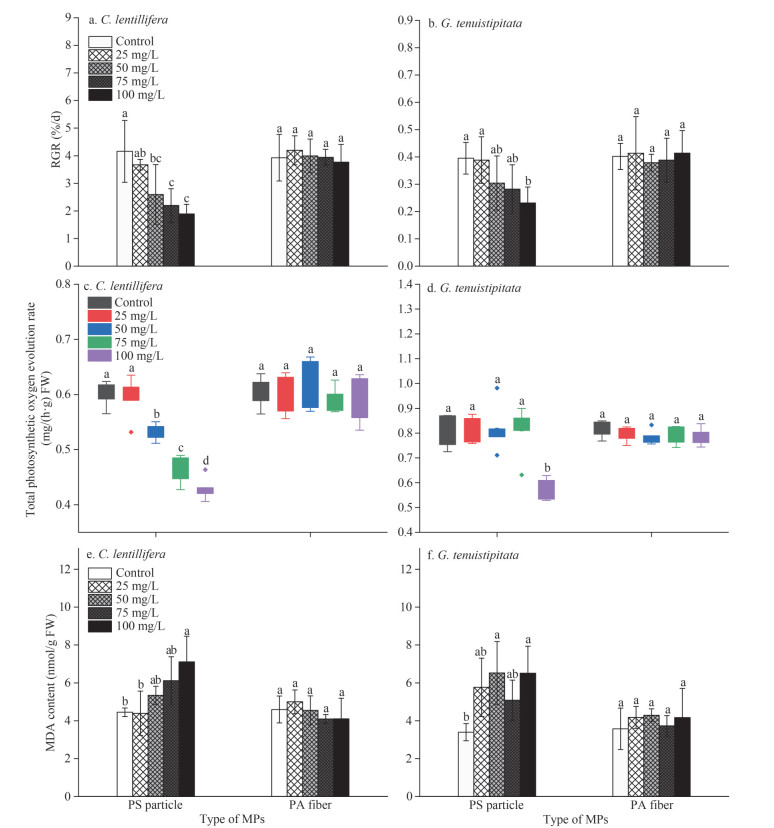
3 RESULT 3.1 Effect of PS particles and PA fibers on the seaweeds 3.1.1 Growth affected by the MPsThe growth of C. lentillifera and G. tenuistipitata was significantly (P < 0.05) inhibited by PS particles, while no inhibitory effect of PA fibers on the growth was observed (Fig. 1a–b). The relative growth rates (RGRs) of C. lentillifera responded negatively to the PS particles concentration (Fig. 1a). For G. tenuistipitata, except at a high concentration (100 mg/L), PS had no significant effect (P > 0.05) on the relative growth rate (Fig. 1b). Compared with the control, when exposed to 100-mg/L PS particles, the growth of C. lentillifera and G. tenuistipitata was inhibited by 54.56% and 30.62%, respectively.
|
| Fig.1 The relative growth rates (RGR), total photosynthetic oxygen evolution rates, and malondialdehyde (MDA) content changes of C. lentillifera (a, c, e) and G. tenuistipitata (b, d, f) exposed to different MPs concentrations In the column chart, means and standard deviation are shown (n=3). The box contains 50% of the data, and the upper and lower edges represent the maximum and minimum values. The points outside the box are "outliers" (n=6). Different letters denote a significant difference in values (P < 0.05). |
An apparent similarity was observed between the levels of photosynthetic oxygen evolution and growth of the two seaweeds treated with MPs (Fig. 1c–d). The PA fibers had no significant (P > 0.05) effect on the photosynthesis of the two seaweeds, but PS with a higher concentration suppressed the total photosynthetic oxygen evolution rate of the seaweeds. The responses of the two seaweeds in photosynthesis were different. For C. lentillifera, when PS particle content was higher than 50 mg/L, the oxygen evolution rate can be inhibited significantly (P < 0.05) (Fig. 2c). Only when G. tenuistipitata was exposed to 100-mg/L PS particle did the oxygen evolution rate decrease (Fig. 2d). Compared with the control, the total photosynthetic oxygen evolution rate of C. lentillifera and G. tenuistipitata decreased by up to 29.19% and 28.23%, respectively.
|
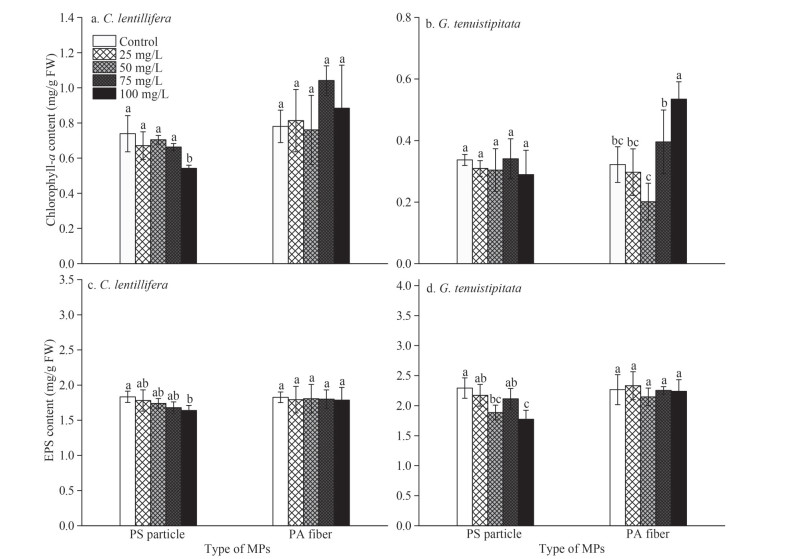
| Fig.2 The contents of chlorophyll-a and extracellular polymeric substances (EPS) of C. lentillifera (a, c) and G. tenuistipitata (b, d) exposed to different MPs concentrations Means and standard deviation are shown (n=3). Different letters denote a significant difference in values (P < 0.05). |
The stress intensity of seaweed caused by MPs was evaluated via the malondialdehyde (MDA) content. Results show that when the seaweeds were exposed to the MPs environment, the changing trend of MDA content was also similar to the growth and the photosynthesis (Fig. 2e–f). All the tested concentrations of PA fibers showed no significant (P > 0.05) effects on MDA content in the two seaweeds. While higher PS concentration treatments induced the MDA content of the seaweeds to increase significantly (P < 0.05). The levels of MDA in C. lentillifera and G. tenuistipitata were highest when exposed to 100-mg/L PS, with the average concentrations of 7.11 and 6.51 nmol/g fresh weight (FW), increasing by 59.89% and 91.79% compared with the control, respectively.
3.1.2 Chlorophyll a and extracellular polymeric substances (EPS) affected by the MPsUnder the condition of 100-mg/L PS, the chlorophyll-a content of C. lentillifera decreased by 39.79% compared with the control (Fig. 2a). No significant difference in chlorophyll-a content in other treated C. lentillifera was observed. For G. tenuistipitata, PS particles have no significant effect on the chlorophyll-a content (P > 0.05; Fig. 2b). However, the chlorophyll-a content of G. tenuistipitata was significantly increased (P < 0.05) under the high concentration of PA fibers (100 mg/ L), increasing by 66.04% compared with the control.
The EPS contents of the C. lentillifera and G. tenuistipitata were decreased with the concentration of PS particles (P < 0.05), but PA fibers did not influence the EPS production of two seaweeds (P > 0.05; Fig. 2c–d). C. lentillifera and G. tenuistipitata had the lowest EPS values at 100-mg/L PS particles, with the average concentrations of 1.64 and 1.77 mg/g FW, decreased by 10.55% and 22.67%, respectively, compared with the control.
3.2 Adsorption and desorption of MPs by seaweedsMPs were found adhered to the surface of C. lentillifera and G. tenuistipitata during the adsorption experiment (Fig. 3). The average adhesion density of PS particles on C. lentillifera and G. tenuistipitata was 114.68 and 546.22 items/cm2 respectively after exposure for 72 h (Fig. 4a). The average adhesion rate of PA fibers on C. lentillifera and G. tenuistipitata were 3.07 and 7.84 items/cm2 respectively (Fig. 4b). The decontamination step demonstrated a significant (P < 0.05) removal effect of MPs from the seaweeds (Fig. 4c–d). Except for PS particles on G. tenuistipitata, the desorption rate of MPs on seaweeds by the washing method was superior to that of the dewatering method, and the combination method of dewatering and washing had the highest MPs removal rate (P < 0.05). For C. lentillifera, the PS density of the surface was reduced to 2.48–13.93 items/cm2 (average 9.59 items/cm2) by the combined strategy of dewatering and washing, which means an average of 91.45% adsorbed PS was removed. By the combined strategy of dewatering and washing, an average of 86.38% PA was removed. For G. tenuistipitata, by the combined strategy, 85.16% adsorbed PS and 87.23% adsorbed PA were desorbed, respectively.
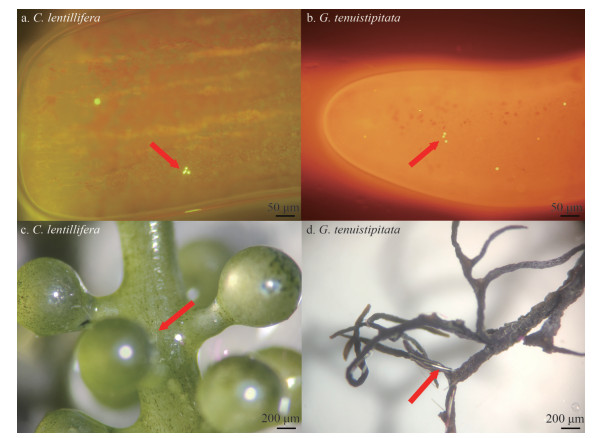
|
| Fig.3 The adherence of PS fluorescent particles (a, b) and PA fibers (c, d) on the surface of C. lentillifera and G. tenuistipitata after 72 h of exposure to MPs examined under the inverted fluorescence microscope and the stereo microscope The red arrows indicate the adhesion position of MPs. |
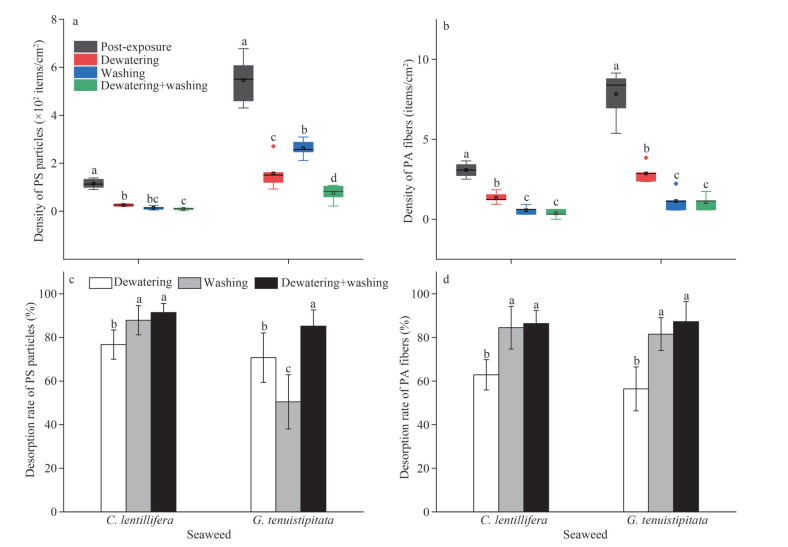
|
| Fig.4 Density of PS particles (a) and PA fibers (b) on the MPs-contaminated of C. lentillifera and G. tenuistipitata, and the desorption rate of PS particles (c) and PA fibers (d) on algal surfaces after dewatering, washing, and combined decontamination In the column chart, means and standard deviation are shown (n=6). In the boxplot, line and square in the box represent the median and mean of the data, respectively. The box contains 50% of the data, and the upper and lower edges represent the maximum and minimum values. The points outside the box are "outliers" (n=6). Different letters denote a significant difference in values (P < 0.05). |
The results of microplastic adhesion density with washing time are shown in the Fig. 5. In Exp.1, at 16 h of washing, the density of PS particles on the surface of C. lentillifera and G. tenuistipitata were 26.41 items/cm2 and 128.52 items/cm2, respectively, which decreased to 19.46% and 21.73% of the untreated samples. The data indicated that over time, more PS particles were eluted from the surface of seaweeds. However, after washing for 4 h, the washing time had no significance on the density of PA fibers (P > 0.05). During the Exp.2 in gradient with elution water, there were no significant differences in the MPs desorption rate of the algal surface in the treatment groups after consuming more than 20 mL of water (P > 0.05) (Fig. 6). In the gradient experiment of washing water consumption and washing time, the results showed that the highest desorption rate of MPs was lower than that of the combined strategy of dewatering and washing.
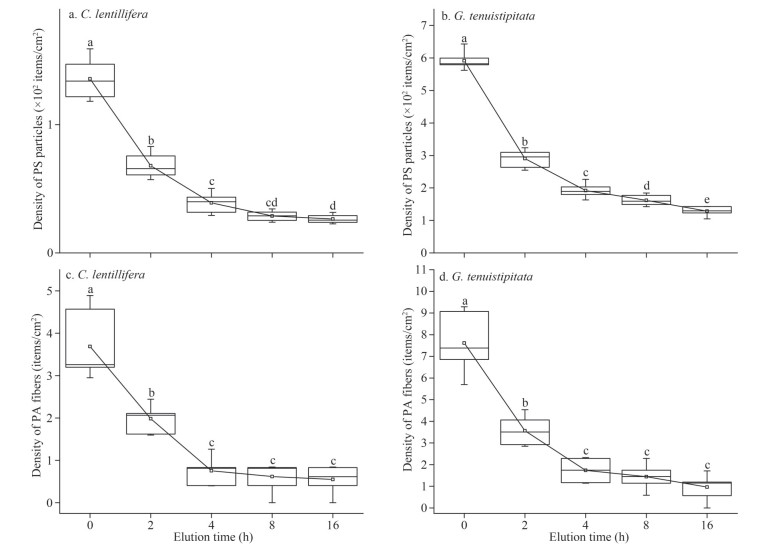
|
| Fig.5 The density of PS fluorescent particles (a, b) and PA fibers (c, d) on the surface of C. lentillifera and G. tenuistipitata washing for 0, 2, 4, 8 and 16 h In the boxplot, line and square in the box represent the median and mean of the data, respectively. The box contains 50% of the data, and the upper and lower edges represent the maximum and minimum values. The means of every treatment are connected by a straight line, and the "outliers" outside the box are hidden (n=6). Different letters denote a significant difference in values (P < 0.05). |

|
| Fig.6 Desorption rates of PS particles (a) and PA fibers (b) on the algal surface under different washing water consumption Means and standard deviation are shown (n=6). Different letters denote a significant difference in values (P < 0.05). |
Microplastics (MPs) are widely distributed in coastal ecosystems, especially in aquaculture areas (Zhang et al., 2020; Campos da Rocha et al., 2021). Therefore, it is necessary to develop technologies to remove MPs from the ocean. Previous research suggested that aquatic plants could remove MPs from freshwater environments through phytoremediation (Kalčíková, 2020; Rozman et al., 2022). Compared to vascular plants, macroalgae (seaweeds) are more suitable for application in marine environments due to the advantages of biomass (Zhang et al., 2022). However, the issue that MPs may impair the growth and physiology of seaweeds requires attention, which is critical for developing bioremediation strategies. In this study, the interaction of MPs with macroalgae was investigated. The effects of growth, photosynthesis, and oxidation degree of two seaweeds were mainly studied.
The results of growth experiment show that the relative growth of C. lentillifera and G. tenuistipitata was inhibited by PS particles (100 mg/L), which is consistent with other studies (Feng et al., 2020a; Khandare et al., 2022). In addition, it was observed that the PS had a negative concentration-dependent effect on the total photosynthetic O2 evolution rate of two seaweeds, which is most likely attributed to the hindered gas exchange by the MPs. The adsorption of MPs on seaweeds may reduce the available light for photosynthesis and could damage the cell wall, which leads to pore formation and consequently enhances the small size of MPs uptake by the seaweeds (Bhattacharya et al., 2010; Yu et al., 2022). Malondialdehyde (MDA) content is usually utilized to describe the degree of membrane peroxidation and the severity of plant reactions when exposed to harsh circumstances (Song et al., 2019). PS particles increased MDA production of C. lentillifera and G. tenuistipitata in this study, indicating that the presence of MPs causes oxidative stress in algae cells. Based on the inhibition of photosynthesis, this result may be attributed to the reduction in the rate of electron transport, which led to a buildup of electrons and consequent oxidative damage to cells (Hong et al., 2009). On the other hand, this study revealed that the high concentration of PS particles had a direct impact on the content of chlorophyll a in C. lentillifera, which is consistent with other published studies (Besseling et al., 2014; Yu et al., 2020). In contrast, the content of chlorophyll a in G. tenuistipitata was not affected by the presence of PS, and the different results may be explained by the various ways that algae express their genes (Song et al., 2020). Extracellular polymeric substances (EPS), which is frequently highlighted as complex high-molecular polymers, is a critical element determining the quantity of MPs adsorbed on the surface of macrophytes (Miao et al., 2009; Sfriso et al., 2021). The results showed that the EPS content of both seaweeds was decreased with the increase of PS particles concentration. Lagarde et al. (2016) reported that the post-transcriptional level of genes involved in exopolysaccharide production was repressed when exposed to high MPs. In addition, the characteristics of EPS and MPs combined into aggregates are also the reason for the low concentration of EPS in the aqueous solution (Cunha et al., 2019; Li et al., 2020b).
On the other hand, the growth, total photosynthetic O2 evolution rate, and the contents of MDA and EPS of two seaweeds were not affected by PA fibers, which means that various MPs types had various effects on macroalgae. PA fibers had no effect on the photosynthetic pigment content of C. lentillifera, but the chlorophyll-a content of G. tenuistipitata was significantly increased. This result may be related to the shading effect of PA fibers. Reducing light intensity appropriately promotes the increase of pigment content in plants, which has been observed in seaweeds growing in the intertidal zone (Sampath-Wiley et al., 2008; Marinho-Soriano, 2012). In this work, the size of PS is much smaller than PA, which may have a negative impact on seaweeds. Specifically, as MP size shrinks compared to algal size, MPs have increasingly adverse effects (Chae et al., 2019; Gao et al., 2021).
Previous research has confirmed that macroalgae can enrich MPs (Feng et al., 2020a, b; Gao et al., 2020; Li et al., 2020a; Peller et al., 2021; Sfriso et al., 2021; Zhang et al., 2022). In this study, we observed that MPs adhered to the cell wall regardless of their chemical composition. The density of MPs adsorbed by G. tenuistipitata was higher than that by C. lentillifera, which may be caused by the high content of EPS in G. tenuistipitata. Recent studies revealed that the level of EPS on the seaweeds surface directly relates to the MPs adhesion (Sfriso et al., 2021). Seng et al. (2020) proposed that EPS and supergene cover could be involved in MPs capture, but the specific mechanism was yet unidentified. Besides, a previous investigation with charged polystyrene beads suggested that these MPs interacted with plant cell walls via electrostatic force (Chi et al., 2018). Microalgae cells, for instance, had a zeta potential of 9.9±5.6 mV, while plain polystyrene had a zeta potential of 18.57±4.13 mV, indicating the existence of significant electrostatic forces (Thiagarajan et al., 2019). Other studies defined the connections between MPs and algal cells as referring to biofilm stickiness or adhesive (Gutow et al., 2016). A recent study hypothesized that exploiting the mechanism of MPs being trapped by macroalgae could be a way to remove MPs from the marine environment (Gao et al., 2021). However, many studies are limited to the in-situ detection of MPs on the surface of marine macroalgae (Seng et al., 2020; Sfriso et al., 2021; Zhang et al., 2022). MPs trapped on macroalgae in the natural environment are either released into the ocean as the seaweeds decayed or accumulated in marine organisms via food webs (Gutow et al., 2016; Feng et al., 2020a). The ability of macroalgae to capture MPs has been confirmed to be universal (Sfriso et al., 2021). Farmed macroalgae has the potential to generate economic value, as well as reduce microplastic pollution in the marine environment. Therefore, the removal of MPs by farmed seaweed has high research value.
Having investigated the effect of cleaning procedures on MPs removal, our results indicate that the amounts of adherent MPs on the algal surface was significantly decreased by the combined dewatering and washing procedures, which is consistent with other studies (Gutow et al., 2016; Sundbæk et al., 2018). The washing process may thin the algae's EPS mucus layer, which helps in particle desorption (Turner et al., 2012). In the washing gradient experiment, the results showed that the majority of weakly adhered MPs were removed by washing with seawater. However, changing washing time and water consumption may not remove the MPs that are strongly adhered to the seaweed surface (Rozman et al., 2022). Meanwhile, the reasons for the detachment of MPs particles and fibers from the algae under water-deficient conditions are currently unclear and require further research. Furthermore, the washing method and dewatering time also affect the removal rate of MPs, which should be considered in future studies.
5 CONCLUSIONThis study investigated the effects of two MPs (PS particles and PA fibers) on two typical commercial macroalgae (Caulerpa lentillifera and Gracilaria tenuistipitata) in Hainan. It was discovered that two types of MPs have different effects on seaweeds. PA fibers had no effect on the growth of two seaweeds, and the addition of PS (100 mg/L) particles significantly inhibits the growth of macroalgae, particularly C. lentillifera (up to 54.56%). In this work, the size of PS is much smaller than PA, which may have a negative impact on seaweeds. Under the stress of the MPs, the tolerance of G. tenuistipitata to PS particles is higher than that of C. lentillifera.
Macroalgae are considered a major temporary sink for MPs, from which a phytoremediation strategy for MPs removal could be developed. The adherence of MPs to macroalgae and the techniques for removing MPs from macroalgae were examined. The density of MPs adsorbed by G. tenuistipitata was higher than that of C. lentillifera, which may be caused by the high content of EPS in G. tenuistipitata. The result of the decontamination step demonstrated that there is a significant loss of adherent particles or fibers by washing and dewatering for all macroalgae, and the combination of dewatering and washing has the highest removal rate of MPs. Our results showed that the growth of C. lentillifera and G. tenuistipitata was not affected at low MPs concentrations, which were much higher than the levels of environmentally relevant concentrations. As a result, macroalgae are worthy of developing a bio-solution for the remediation of MPs in the marine environment.
6 DATA AVAILABILITY STATEMENTAll data in the current study are available upon request, according to the authors.
7 ACKNOWLEDGMENTWe are extremely thankful to Hainan University for providing the necessary facilities. We also thank Prof. Zhiyuan LIU, College of Marine Sciences, Hainan University, for her assistance in correcting this article.
Akdogan Z, Guven B. 2019. Microplastics in the environment: a critical review of current understanding and identification of future research needs. Environmental Pollution, 254: 113011.
DOI:10.1016/j.envpol.2019.113011 |
Besseling E, Wang B, Lürling M, et al. 2014. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environmental Science & Technology, 48(20): 12336-12343.
DOI:10.1021/es503001d |
Bhattacharya P, Lin S J, Turner J P, et al. 2010. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. The Journal of Physical Chemistry C, 114(39): 16556-16561.
DOI:10.1021/jp1054759 |
Campos da Rocha F O, Martinez S T, Campos V P, et al. 2021. Microplastic pollution in Southern Atlantic marine waters: review of current trends, sources, and perspectives. Science of the Total Environment, 782: 146541.
DOI:10.1016/j.scitotenv.2021.146541 |
Chae Y, Kim D, An Y J. 2019. Effects of micro-sized polyethylene spheres on the marine microalga Dunaliella salina: focusing on the algal cell to plastic particle size ratio. Aquatic Toxicology, 216: 105296.
DOI:10.1016/j.aquatox.2019.105296 |
Chen G L, Feng Q Y, Wang J. 2020a. Mini-review of microplastics in the atmosphere and their risks to humans. Science of the Total Environment, 703: 135504.
DOI:10.1016/j.scitotenv.2019.135504 |
Chen S W, Xu K, Ji D H, et al. 2020b. Release of dissolved and particulate organic matter by marine macroalgae and its biogeochemical implications. Algal Research, 52: 102096.
DOI:10.1016/j.algal.2020.102096 |
Chi Y Z, Li Y P, Zhang G L, et al. 2018. Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carbohydrate Polymers, 181: 616-623.
DOI:10.1016/j.carbpol.2017.11.104 |
Cunha C, Faria M, Nogueira N, et al. 2019. Marine vs freshwater microalgae exopolymers as biosolutions to microplastics pollution. Environmental Pollution, 249: 372-380.
DOI:10.1016/j.envpol.2019.03.046 |
Eriksen M, Lebreton L C M, Carson H S, et al. 2014. Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250, 000 tons afloat at sea. PLoS One, 9(12): e111913.
DOI:10.1371/journal.pone.0111913 |
Feng Z H, Zhang T, Shi H H, et al. 2020a. Microplastics in bloom-forming macroalgae: distribution, characteristics and impacts. Journal of Hazardous Materials, 397: 122752.
DOI:10.1016/j.jhazmat.2020.122752 |
Feng Z H, Zhang T, Wang J X, et al. 2020b. Spatio-temporal features of microplastics pollution in macroalgae growing in an important mariculture area, China. Science of the Total Environment, 719: 137490.
DOI:10.1016/j.scitotenv.2020.137490 |
Figueroa F L, Conde- Álvarez R, Gómez I. 2003. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynthesis Research, 75(3): 259-275.
DOI:10.1023/a:1023936313544 |
Gao F L, Li J X, Hu J, et al. 2020. Occurrence of microplastics carried on Ulva prolifera from the Yellow Sea, China. Case Studies in Chemical and Environmental Engineering, 2: 100054.
|
Gao G, Gao L, Jiang M J, et al. 2022. The potential of seaweed cultivation to achieve carbon neutrality and mitigate deoxygenation and eutrophication. Environmental Research Letters, 17(1): 014018.
DOI:10.1088/1748-9326/ac3fd9 |
Gao G, Zhao X, Jin P, et al. 2021. Current understanding and challenges for aquatic primary producers in a world with rising micro- and nano-plastic levels. Journal of Hazardous Materials, 406: 124685.
DOI:10.1016/j.jhazmat.2020.124685 |
Glenn E P, Doty M S. 1992. Water motion affects the growth rates of Kappaphycus alvarezii and related red seaweeds. Aquaculture, 108(3-4): 233-246.
DOI:10.1016/0044-8486(92)90109-x |
Goss H, Jaskiel J, Rotjan R. 2018. Thalassia testudinum as a potential vector for incorporating microplastics into benthic marine food webs. Marine Pollution Bulletin, 135: 1085-1089.
DOI:10.1016/j.marpolbul.2018.08.024 |
Gutow L, Eckerlebe A, Giménez L, et al. 2016. Experimental evaluation of seaweeds as a vector for microplastics into marine food webs. Environmental Science & Technology, 50(2): 915-923.
DOI:10.1021/acs.est.5b02431 |
Guzzetti E, Sureda A, Tejada S, et al. 2018. Microplastic in marine organism: environmental and toxicological effects. Environmental Toxicology and Pharmacology, 64: 164-171.
DOI:10.1016/j.etap.2018.10.009 |
Hassid W Z, Abraham S K. 1957. Chemical procedures for analysis of polysaccharides. Methods in Enzymology, 3: 34-50.
DOI:10.1016/S0076-6879(57)03345-5 |
Haward M. 2018. Plastic pollution of the world's seas and oceans as a contemporary challenge in ocean governance. Nature Communications, 9(1): 667.
DOI:10.1038/s41467-018-03104-3 |
Hazeem L J, Yesilay G, Bououdina M, et al. 2020. Investigation of the toxic effects of different polystyrene micro-and nanoplastics on microalgae Chlorella vulgaris by analysis of cell viability, pigment content, oxidative stress and ultrastructural changes. Marine Pollution Bulletin, 156: 111278.
DOI:10.1016/j.marpolbul.2020.111278 |
Hong Y, Hu H Y, Xie X, et al. 2009. Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquatic Toxicology, 91(3): 262-269.
DOI:10.1016/j.aquatox.2008.11.014 |
Kalčíková G. 2020. Aquatic vascular plants—a forgotten piece of nature in microplastic research. Environmental Pollution, 262: 114354.
DOI:10.1016/j.envpol.2020.114354 |
Kalčíková G, Skalar T, Marolt G, et al. 2020. An environmental concentration of aged microplastics with adsorbed silver significantly affects aquatic organisms. Water Research, 175: 115644.
DOI:10.1016/j.watres.2020.115644 |
Khandare S D, Chaudhary D R, Jha B. 2022. Marine bacteria-based polyvinyl chloride (PVC) degradation by-products: toxicity analysis on Vigna radiata and edible seaweed Ulva lactuca. Marine Pollution Bulletin, 175: 113366.
DOI:10.1016/j.marpolbul.2022.113366 |
Lagarde F, Olivier O, Zanella M, et al. 2016. Microplastic interactions with freshwater microalgae: hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environmental Pollution, 215: 331-339.
DOI:10.1016/j.envpol.2016.05.006 |
Lebreton L C M, van der Zwet J, Damsteeg J W, et al. 2017. River plastic emissions to the world's oceans. Nature Communications, 8: 15611.
DOI:10.1038/ncomms15611 |
Li Q P, Feng Z H, Zhang T, et al. 2020a. Microplastics in the commercial seaweed nori. Journal of Hazardous Materials, 388: 122060.
DOI:10.1016/j.jhazmat.2020.122060 |
Li S X, Wang P P, Zhang C, et al. 2020b. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Science of the Total Environment, 714: 136767.
DOI:10.1016/j.scitotenv.2020.136767 |
Liu W Y, Zhang J L, Liu H, et al. 2021. A review of the removal of microplastics in global wastewater treatment plants: characteristics and mechanisms. Environment International, 146: 106277.
DOI:10.1016/j.envint.2020.106277 |
Marinho-Soriano E. 2012. Effect of depth on growth and pigment contents of the macroalgae Gracilaria bursa-pastoris. Revista Brasileira de Farmacognosia, 22(4): 730-735.
DOI:10.1590/s0102-695x2012005000059 |
Mateos-Cárdenas A, Scott D T, Seitmaganbetova G, et al. 2019. Seitmaganbetova G et al. 2019. Polyethylene microplastics adhere to Lemna minor (L.), yet have no effects on plant growth or feeding by Gammarus duebeni (Lillj.). Science of the Total Environment, 689: 413-421.
DOI:10.1016/j.scitotenv.2019.06.359 |
Miao A J, Schwehr K A, Xu C, et al. 2009. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environmental Pollution, 157(11): 3034-3041.
DOI:10.1016/j.envpol.2009.05.047 |
Miller M E, Hamann M, Kroon F J. 2020. Bioaccumulation and biomagnification of microplastics in marine organisms: a review and meta-analysis of current data. PLoS One, 15(10): e0240792.
DOI:10.1371/journal.pone.0240792 |
Pancha I, Chokshi K, Maurya R, et al. 2015. Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresource Technology, 189: 341-348.
DOI:10.1016/j.biortech.2015.04.017 |
Peller J, Nevers M B, Byappanahalli M, et al. 2021. Sequestration of microfibers and other microplastics by green algae, Cladophora, in the US Great Lakes. Environmental Pollution, 276: 116695.
DOI:10.1016/j.envpol.2021.116695 |
Perren W, Wojtasik A, Cai Q. 2018. Removal of microbeads from wastewater using electrocoagulation. ACS Omega, 3(3): 3357-3364.
DOI:10.1021/acsomega.7b02037 |
Rozman U, Jemec Kokalj A, Dolar A, et al. 2022. Long-term interactions between microplastics and floating macrophyte Lemna minor: the potential for phytoremediation of microplastics in the aquatic environment. Science of the Total Environment, 831: 154866.
DOI:10.1016/j.scitotenv.2022.154866 |
Rozman U, Turk T, Skalar T, et al. 2021. An extensive characterization of various environmentally relevant microplastics—material properties, leaching and ecotoxicity testing. Science of the Total Environment, 773: 145576.
DOI:10.1016/j.scitotenv.2021.145576 |
Sampath-Wiley P, Neefus C D, Jahnke L S. 2008. Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: fluctuations in antioxidant contents, photosynthetic pigments and photosynthetic efficiency in the red alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). Journal of Experimental Marine Biology and Ecology, 361(2): 83-91.
DOI:10.1016/j.jembe.2008.05.001 |
Seng N, Lai S, Fong J, et al. 2020. Early evidence of microplastics on seagrass and macroalgae. Marine and Freshwater Research, 71(8): 922-928.
DOI:10.1071/mf19177 |
Sfriso A A, Tomio Y, Juhmani A S, et al. 2021. Macrophytes: a temporary sink for microplastics in transitional water systems. Water, 13(21): 3032.
DOI:10.3390/w13213032 |
Song C F, Liu Z Z, Wang C L, et al. 2020. Different interaction performance between microplastics and microalgae: the bio-elimination potential of Chlorella sp. L38 and Phaeodactylum tricornutum MASCC-0025. Science of the Total Environment, 723: 138146.
DOI:10.1016/j.scitotenv.2020.138146 |
Song C F, Wei Y L, Qiu Y T, et al. 2019. Biodegradability and mechanism of florfenicol via Chlorella sp. UTEX1602 and L38: experimental study. Bioresource Technology, 272: 529-534.
DOI:10.1016/j.biortech.2018.10.080 |
Sundbæk K B, Koch I D W, Villaro C G, et al. 2018. Sorption of fluorescent polystyrene microplastic particles to edible seaweed Fucus vesiculosus. Journal of Applied Phycology, 30(5): 2923-2927.
DOI:10.1007/s10811-018-1472-8 |
Talvitie J, Mikola A, Koistinen A, et al. 2017. Solutions to microplastic pollution—removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Research, 123: 401-407.
DOI:10.1016/j.watres.2017.07.005 |
Taylor R B, Cole R G. 1994. Mobile epifauna on subtidal brown seaweeds in Northeastern New Zealand. Marine Ecology Progress Series, 115: 271-282.
|
Thiagarajan V, Pavani M, Archanaa S, et al. 2019. Diminishing bioavailability and toxicity of P25 TiO2 NPs during continuous exposure to marine algae Chlorella sp. Chemosphere, 233: 363-372.
DOI:10.1016/j.chemosphere.2019.05.270 |
Turner A, Brice D, Brown M T. 2012. Interactions of silver nanoparticles with the marine macroalga, Ulva lactuca. Ecotoxicology, 21(1): 148-154.
DOI:10.1007/s10646-011-0774-2 |
Wellburn A R. 1994. The Spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology, 144(3): 307-313.
DOI:10.1016/s0176-1617(11)81192-2 |
Wright S L, Thompson R C, Galloway T S. 2013. The physical impacts of microplastics on marine organisms: a review. Environmental Pollution, 178: 483-492.
DOI:10.1016/j.envpol.2013.02.031 |
Wu Y M, Guo P Y, Zhang X Y, et al. 2019. Effect of microplastics exposure on the photosynthesis system of freshwater algae. Journal of Hazardous Materials, 374: 219-227.
DOI:10.1016/j.jhazmat.2019.04.039 |
Xu S, Ma J, Ji R, et al. 2020. Microplastics in aquatic environments: occurrence, accumulation, and biological effects. Science of the Total Environment, 703: 134699.
DOI:10.1016/j.scitotenv.2019.134699 |
Yaseen A, Assad I, Sofi M S, et al. 2022. A global review of microplastics in wastewater treatment plants: understanding their occurrence, fate and impact. Environmental Research, 212: 113258.
DOI:10.1016/j.envres.2022.113258 |
Yu H W, Qi W X, Cao X F, et al. 2022. Impact of microplastics on the foraging, photosynthesis and digestive systems of submerged carnivorous macrophytes under low and high nutrient concentrations. Environmental Pollution, 292: 118220.
DOI:10.1016/j.envpol.2021.118220 |
Yu H W, Zhang X L, Hu J W, et al. 2020. Ecotoxicity of polystyrene microplastics to submerged carnivorous Utricularia vulgaris plants in freshwater ecosystems. Environmental Pollution, 265: 114830.
DOI:10.1016/j.envpol.2020.114830 |
Zhang Q Z, Diao X P, Xie J, et al. 2020. Distribution characteristics of microplastics in water and sediment of mariculture area in Eastern Hainan. Natural Science Journal of Hainan University, 38(2): 159-165.
(in Chinese with English abstract) DOI:10.15886/j.cnki.hdxbzkb.2020.0023 |
Zhang T, Wang J X, Liu D X, et al. 2022. Loading of microplastics by two related macroalgae in a sea area where gold and green tides occur simultaneously. Science of the Total Environment, 814: 152809.
DOI:10.1016/j.scitotenv.2021.152809 |
Zhang Z Q, Chen Y G. 2020. Effects of microplastics on wastewater and sewage sludge treatment and their removal: a review. Chemical Engineering Journal, 382: 122955.
DOI:10.1016/j.cej.2019.122955 |
 2023, Vol. 41
2023, Vol. 41


