Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Xiaoyan, TIAN Yuan, YU Haohui, CAO Min, LI Chao
- Genome-wide characterization of mapk gene family in black rockfish Sebastes schlegelii and their expression patterns against Edwardsiella piscicida infection
- Journal of Oceanology and Limnology, 41(6): 2348-2362
- http://dx.doi.org/10.1007/s00343-023-2314-3
Article History
- Received Sep. 15, 2022
- accepted in principle Nov. 1, 2022
- accepted for publication Nov. 16, 2022
2 Key Laboratory of Mariculture (Ocean University of China), Ministry of Education, Ocean University of China, Qingdao 266003, China
Mitogen-activated protein kinases (MAPKs) are class of serine/threonine protein kinases that activated by extracellular stimuli through the MAPK cascade that widely presented in most cells (Seger and Krebs, 1995; Cowan and Storey, 2003; Roux and Blenis, 2004; Sun et al., 2015). Usually, MAPKs mainly contain extracellular signal-regulated kinases (ERK), c-Jun amino-terminal kinases (JNK), and p38-subfamily, those were classified according to the differences in dual-phosphorylation sites (Krens et al., 2006). Being well known, phosphorylated MAPKs can transduce extracellular signals into intracellular responses, activate downstream molecules, and regulate the expressions of target genes in various biological processes such as growth, development, apoptosis, and responses to various environmental stresses and bacterial infections (Garrington and Johnson, 1999; Cowan and Storey, 2003; Kyriakis and Avruch, 2012). Accordingly, numerous literatures focused on the structures and functions of mapk genes in both mammals and teleost. For instance, it was reported that ERK could be activated by oxidant stress in kidney of mouse (Mus musculus) (Arany et al., 2005); ERK2, ERK3, and p38A were expressed at constant levels during zebrafish embryogenesis as proved by semi-quantitative reverse transcriptase-PCR analysis and in-situ hybridization (Krens et al., 2006). Tian et al. (2019) identified 14 mapk genes in spotted sea bass (Lateolabrax maculatus), and speculated that mapk genes such as JNK (JNK1, JNK2) and p38 (p38β, p38a, and p38b) were involved in the response to salinity and hypoxia challenges.
Additionally, many studies have clarified the functions of mapk genes in immune response ranging from mammals to teleost. In mammalian species, mapk genes were believed closely associated with the innate immunity and adaptive immunity (Dong et al., 2002). For teleost, the conserved mapk genes were considered as critical mediators of innate immune system for regulating the expression of cytokines to defend against various types of pathogen infection (Zhu et al., 2014; Jia et al., 2015; Umasuthan et al., 2015; Sun et al., 2020). For example, the injection of Aeromonas hydrophila and muramyl dipeptide in the intestine of grass carp (Ctenopharyngodon idella) can induce the expression of Cip38 and Cip38β, which also can influence the expression of inflammatory cytokines and antimicrobial peptides. This study proved the crucial roles of p38 genes in bacteria-induced intestinal inflammation (Sun et al., 2020). Moreover, we found that ERK genes elicit a strong immune response to the stimulation of polyI꞉C and flagellin in large yellow croaker (Larimichthys crocea) (Jia et al., 2015). Similar findings were also observed in other teleosts, such as Atlantic salmon (Salmo salar) (Hansen and Jørgensen, 2007), grouper rock bream (Oplegnathus fasciatus) (Umasuthan et al., 2015), orange-spotted grouper (Epinephelus coioides) (Sun et al., 2017), and blunt snout bream (Megalobrama amblycephala) (Zhang et al., 2019). These published studies demonstrated that mapk genes participated in the immune response and the regulation of defensing activities in teleost (Menon et al., 2017; He et al., 2018; Zhang et al., 2019; Cheng et al., 2020; Wei et al., 2020).
Black rockfish (Sebastes schlegelii) is an economically important mariculture species in the northern China. Expansion of aquaculture industry due to market demand has resulted in frequent outbreaks of various bacterial diseases, causing serious economic losses to the farming industry (Kitamura et al., 2006; Cao et al., 2020). Studying the antibacterial mechanism of black rockfish is beneficial to disease prevention. To investigate the potential roles of mapk genes in black rockfish upon infection with pathogens, a systematically identification and characterization of mapk genes based on genome and transcriptome were performed in this study. Their characteristics, phylogenetic relationships, and selective pressures were analyzed. Moreover, the expression patterns of 15 mapk genes in healthy tissues of black rockfish were detected by qRT-PCR, as well as their response expressions following Edwardsiella piscicida infection, aiming to provide a fundamental resource to understand the potential immune functions of mapk genes of black rockfish.
2 MATERIAL AND METHOD 2.1 Identification of mapk genes of black rockfishTo identify mapk genes of black rockfish, we firstly downloaded several sequences of MAPKs from available species including human (Homo sapiens), mouse (Mus musculus), chicken (Gallus gallus), and several teleost from the NCBI database.These genes were chosen as queries for searching the candidates of mapk genes using TBLASTN. Thus, the candidate mapk genes from black rockfish were obtained against available whole genome sequence and transcriptome sequences with E-value less than 1e-10. Meanwhile, we confirmed these genes by predicting their open reading frame (ORFs) using the ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) and their NR annotations.
2.2 Phylogenetic relationships and collinearity analysisThe amino acid sequences of mapk genes from several representative vertebrates including human, mouse, chicken, cow (Bos taurus), frog (Ceratophrys cornuta), zebrafish, Nile tilapia (Oreochromis niloticus), Atlantic salmon, large yellow croaker, torafugu (Takifugu rubripes), and Japanese medaka (Oryzias latipes) that were downloaded from the NCBI database, and those that were identified in this study were aligned, to clarify the evolutionary relationships of mapk genes in black rockfish. In detail, software MUSCLE (MUltiple Sequence Comparison by Log-Expectation) was employed to perform multiple amino acid sequences (Edgar, 2004). Subsequently, software MEGA 7 was used to construct the neighbor-joining (NJ) phylogenetic tree with 1 000 bootstraps (Kumar et al., 2016). Finally, the iTOL (the Interactive Tree of Life) was used to display and embellish the tree (http://itol.embl.de/).
In addition, collinearity analysis was performed to confirm the accuracy of identification and annotation of these mapk genes, especially for the duplicated ones. The neighboring genes around mapk genes in black rockfish were identified from its genome. The genomic information of zebrafish was obtained from NCBI, Genomicus, and Ensembl databases. Meanwhile, annotation and their chromosome locations of these mapk genes were obtained from the genome of black rockfish. At last, software TBtools was used to present the chromosomal locations of mapk genes (Chen et al., 2020).
2.3 Gene structure, domain, and motif analysisThe information about the numbers of exon-intron, functional domains and motifs per mapk gene of black rockfish were presented with TBtools (Chen et al., 2020). Molecular weights and theoretical isoelectric point (pI) of these proteins was calculated using ProtParam. Information about gene structures was extracted from the Nr annotation results. In addition, SMRT database was utilized to predict the architectures of the typical domain of these mapk genes. The conserved protein motifs of each mapk gene were identified with MEME (Bailey et al., 2009), then were annotated by InterProScan (Mulder and Apweiler, 2007). Additionally, we compared the copy numbers of mapks in vertebrates including black rockfish.
2.4 Selective pressure analysisTo study the selective pressures on these mapk genes during evolution, different amino acid sequences of each mapk gene from human, mouse, chicken, cow, frog, Atlantic salmon, zebrafish, torafugu, Japanese medaka, and black rockfish were firstly aligned using software MUSCLE (Edgar, 2004). Subsequently, we used the PAL2NAL program to perform the codon alignments in coding DNA in the estimation of type and ratio of nucleotide substitutions. Meanwhile, the NJ tree of each mapk gene was constructed according to the aligned amino acid sequences using MEGA 7 software with the JTT model. Thus, dN/dS with branch-site model were compared to generate the selective pressure of each mapk gene among these species using PAML program (Yang, 2007). P < 0.05 was chosen to assess the significance of the alternative hypothesis.
2.5 Sample collection from the healthy tissues of S. schlegeliiAll procedures involving the handling and treatment of fish in this study were approved by the Institutional Animal Care and Use Committee, Qingdao Agricultural University. The individuals of black rockfish (body length 15±0.55 cm and body weight 110±5 g) were sampled from Weihai, Shandong, China, and cultured in laboratory for one week before experiment. During the period of experiment, the environmental conditions were kept relatively constant. To understand the expression levels of these mapk genes in healthy tissues of S. schlegelii, eight tissues (brain, gill, muscle, kidney, liver, spleen, intestine, and skin) were collected to detect the expression patterns of 15 mapk genes. During sampling collection, individuals were anesthetized with MS-222 (Sigma Aldrich Co., St. Louis, MO, USA). After that, eight tissues were collected separately from 9 fish for RNA extraction. Tissues from three fish individuals were mixed as one sample.
2.6 Bacterial infection experimentFor exploring the expression profiles of mapk genes in black rockfish following E. piscicida infection, this bacterium was firstly isolated from diseased fish. Then, E. piscicida was cultured in LB medium with 180 r/min at 28 ℃. The concentration of E. piscicida used in the experiment was calculated by counting colony forming unit (CFU) onto plates. Finally, the individuals immersed 4 h in E. piscicida with a final concentration of 1×107 CFU/mL. In contrast, individuals in seawater were collected as control. Subsequently, skin, gill, and intestine were collected at 0 h, 2 h, 6 h, 12 h, 24 h, and 48 h time points. Then, the collected samples were stored in liquid nitrogen for RNA extraction.
2.7 RNA extraction, cDNA synthesis, and qRT-PCR experimentTrizol method (Invitrogen, Carlsbad, CA, USA) was used to extract RNA from different tissues, including 8 healthy tissues, mucosal tissues following E. piscicida infection. After extraction, RNase-Free DNase I (TIANGEN, Beijing, China) was added into the extracted RNA to digest the contaminating DNA according to the manufacturer's instructions. Afterthat, 1% agarose gels and Biodropsis BD-1000 nucleic acid analyzer (OSTC, Beijing) were used to detected the integrity and concentration of the extracted RNA, respectively. RNA with A260/280 and A260/230 over 1.8 were used in this study. Then, 1-μg RNA was reverse transcribed into cDNA synthesis using PrimeScriptTM RT reagent Kit (TaKaRa, Japan) following the manufacturer's instructions. Subsequently, qRT-PCR was used to detect the expression levels of mapk genes in 8 healthy tissues and 3 mucosal tissues (skin, gill, and intestine) following E. piscicida infection in black rockfish. The EF1A was selected as a house-keeping gene for normalization (Ma et al., 2013). The primers used in this study were designed using software Primer 5 and primers are listed in Supplementary Table S1. Before experiment, the amplification efficiency of each primer was confirmed. In detail, the target amplicon of each primer was collected from gel extraction. Then, the purified PCR amplicon was cloned into pMD19-T vector and transformed into Escherichia coli, followed by plasmid extraction.Then, the 10-fold serial diluted standard plasmid DNAs were used as templates to generate standard curve and calculate the amplification efficiency of each primer. Those with amplification efficiency from 90% to 105% were used for qRT-PCR experiment. After validation, qRT-PCR experiment was performed on CFX96 real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA). Three biological replicates and technical replicates for each sample were performed. Finally, the relative expression levels were calculated using the 2-ΔΔCt method (Livak and Schmittgen, 2001). The relative expression levels of the detected genes in qRT-PCR were presented as mean±standard deviation (SD). Software SPSS Statistical v17.0 was used to perform statistical analysis. P < 0.05 presented significant differences between samples.
3 RESULT 3.1 Characterization of mapk genes in black rockfishTotally, 15 mapk genes were identified in black rockfish based on available databases. The detailed characteristics of these mapk genes are summarized in Table 1. Based on the dual-phosphorylation sites, these mapk genes can be divided into three subfamilies: mapk1, mapk3, mapk4, mapk6, mapk7, and mapk15 belonging to ERK subfamily; mapk8a, mapk8b, mapk9, and mapk10 belonging to JNK subfamily; and mapk11, mapk12, mapk13, mapk14a, and mapk14b belonging to p38 subfamily. In detail, ERK, JNK, and p38 harbored the typical amino-acid residues Thr-Glu-Tyr, Thr-Pro-Tyr, and Thr-Gly-Tyr, respectively. Additionally, the lengths of the amino acids of these mapk genes were ranged from 345 to 1 123 bp, the values of molecular weight (MW) varied from 39.75 to 122.75 kDa, and the pIs varied from 4.93 to 9.03.
Copy numbers of mapk genes were examined in several high vertebrates, teleosts including black rockfish (Fig. 1). Overall, the copy numbers were relatively conserved in the selected species, ranging from 10 to 15. For instance, 13, 13, 10, 12–15 members of mapk genes have been identified in human, mouse, chicken, and teleosts, respectively. Most mapk genes were present with a single copy, while duplicated mapk8 and mapk14 genes were detected in several teleost, including zebrafish, large yellow croaker, spotted sea bass, turbot, and black rockfish. Thus, we speculated that the duplication events of mapk genes seem to be teleost-specific.
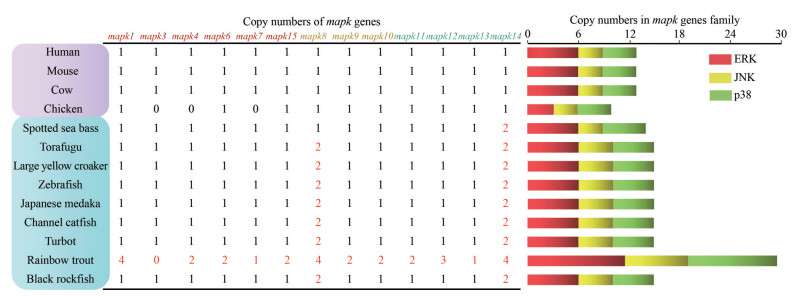
|
| Fig.1 Copy numbers of mapk genes in 13 representative vertebrates Different color shades represent the different groups: higher vertebrates are marked by purple, and teleosts are shown in blue. The copy number of three mapk subfamilies were represented by colored rectangle: ERK in red, JNK in yellow, and p38 in green. |
For phylogenetic tree construction, amino acid sequences of mapk genes from black rockfish and several species were selected. Results show that these mapk genes are clustered with respective counterparts as expected and clearly divided into three clusters containing ERK, JNK, and p38, which were consistent with the classification of mapk families (Fig. 2). The results show the accuracy of the annotation and classification of mapk genes in black rockfish.
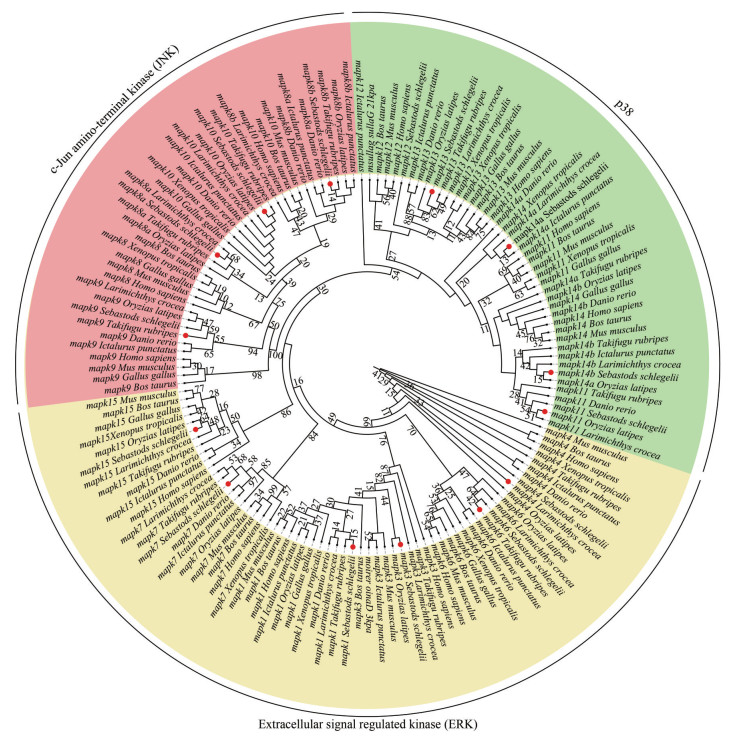
|
| Fig.2 Phylogenetic analysis of mapk genes from the representative vertebrates The amino acid sequences of mapk genes were used to construct a neighbor-joining phylogenetic tree with 1 000 bootstrap replications using MEGA 7 software. Different subfamilies are represented by colored arcs and mapk genes of black rockfish are marked with red dots. |
Syntenic analysis was performed for further confirmation of mapk annotation, especially for those with duplicated copies (Fig. 3; Supplementary Table S2). It is obvious that the syntenic blocks surrounding the tested mapk8a, mapk8b, mapk14a, and mapk14b are similar between zebrafish and black rockfish. This result also confirms the identification and annotation of duplicated mapk genes in black rockfish. Moreover, in zebrafish and black rockfish, there were several paralogous genes located in the neighboring regions of duplicated mapk8 and mapk14, such as vstm4a, vstm4b, gdf10a, gdf10b, antxrld, antxrldc, srpk1a, and srpk1b. It demonstrates that these duplicated mapk genes could be derived from the events of whole-genome duplication.
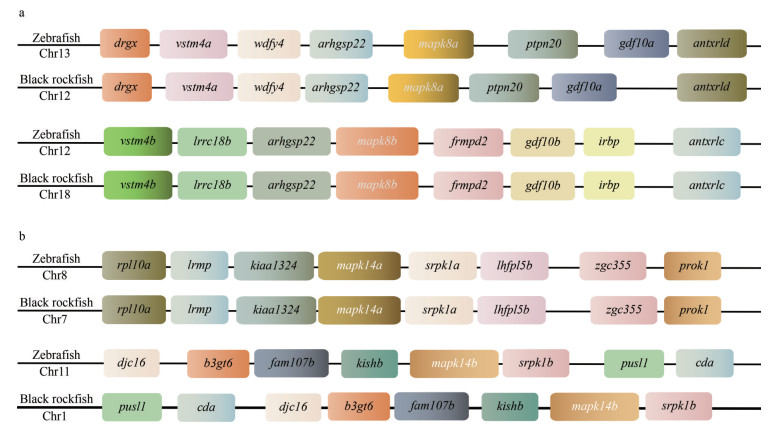
|
| Fig.3 Syntenic analysis of duplicated mapk8 and mapk14 genes in zebrafish and black rockfish Full names of these genes are provided in Supplementary Table S2. a. syntenic analysis of duplicated mapk8 in zebrafish and black rockfish; b. syntenic analysis of duplicated mapk14 genes in zebrafish and black rockfish. |
Chromosomal distribution analysis show that these 15 mapk genes were unevenly distributed on 11 chromosomes (Chrs) of black rockfish. For example, Chr1, Chr2, Chr5, Chr6, Chr7, Chr8, Chr12, Chr14, Chr16, Chr18, and Chr12. Chr1, Chr6, Chr7, and Chr18 harbored 2 mapk genes, while the other 7 mapk genes were located on different chromosomes separately (Fig. 4). Meanwhile, the gene structures such as organization of exon-intron in these mapk genes were examined in black rockfish. As shown in Fig. 5a, the exon numbers of 15 mapk genes varied from 5 to 13, and gene members from same subfamily shared similar exon number. Specifically, most genes from ERK subfamily possessed 5–7 exons, except for mapk15 with 13 exons. In JNK subfamily, gene structures of mapk8a, mapk8b, mapk9, and mapk10, were all comprised of 10 exons. In addition, 11 exons were observed in four p38 genes (mapk11, mapk12, mapk14a, and mapk14b), while the other p38 gene, mapk13, contained only 5 exons. The diverse exon-intron structures of mapk genes from different subfamilies may be related to their distinct biological functions. The S_TKc domains were detected on each of MAPK proteins in black rockfish (Fig. 5b). A majority of MAPK proteins contained low complexity regions, while coiled coil region was present in mapk7 only. Motifs were identified to further interpret the structural diversity of mapk genes in black rockfish. It is obvious that these mapk genes displayed similar motif arrangements (Fig. 5c). In detail, motifs 1–6 and 9– 10 distributed in all the identified mapk genes of black rockfish, except for mapk4, mapk6, and mapk11. Motif 7 was found in the mapk members of JNK subfamily only. However, motif 8 was present in ERK and p38 subfamilies mainly. These specific motifs may be related to the functions of different mapk subfamily genes in black rockfish.

|
| Fig.4 Chromosomal distribution and duplication modes among mapk genes of black rockfish The gray lines represented the syntenic blocks across the genome. |
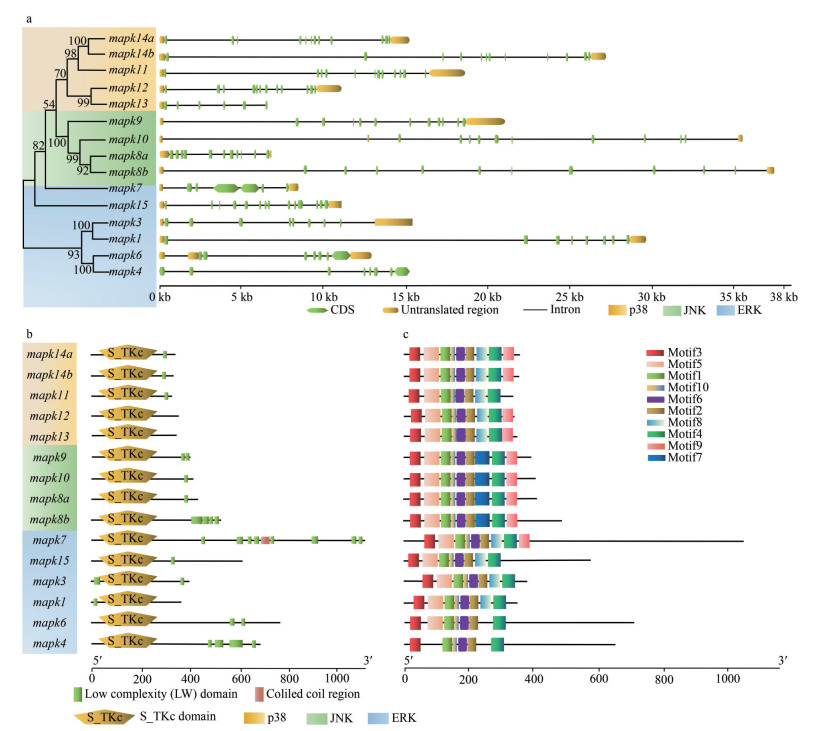
|
| Fig.5 Gene structure, functional domain, and conserved motif analyses of mapk genes in black rockfish a. the gene structure of mapk genes in black rockfish. The phylogenetic tree was constructed based on the neighbor-joining method and JTT model with 1 000 bootstrap replicates using software MEGA 7.0. Branch shades represent the different groups: p38 in yellow, JNK in green, and ERK in blue. Green and yellow boxes represented the exons and UTRs (untranslated regions), respectively; b. functional domains of mapk genes in black rockfish. The domains were analyzed using SMART online tools; c. motif structure of mapk genes in black rockfish. The conserved motifs were determined using software MEME and marked with various colors. |
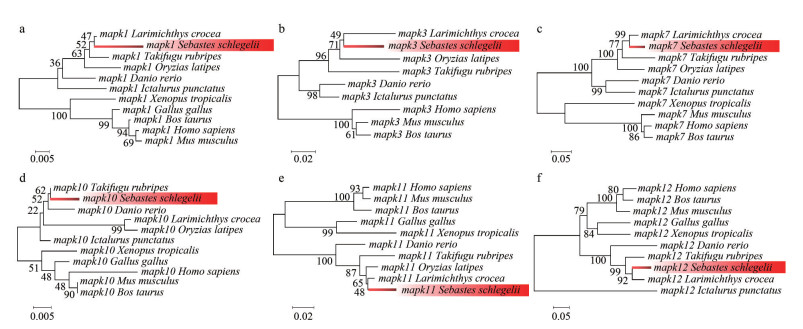
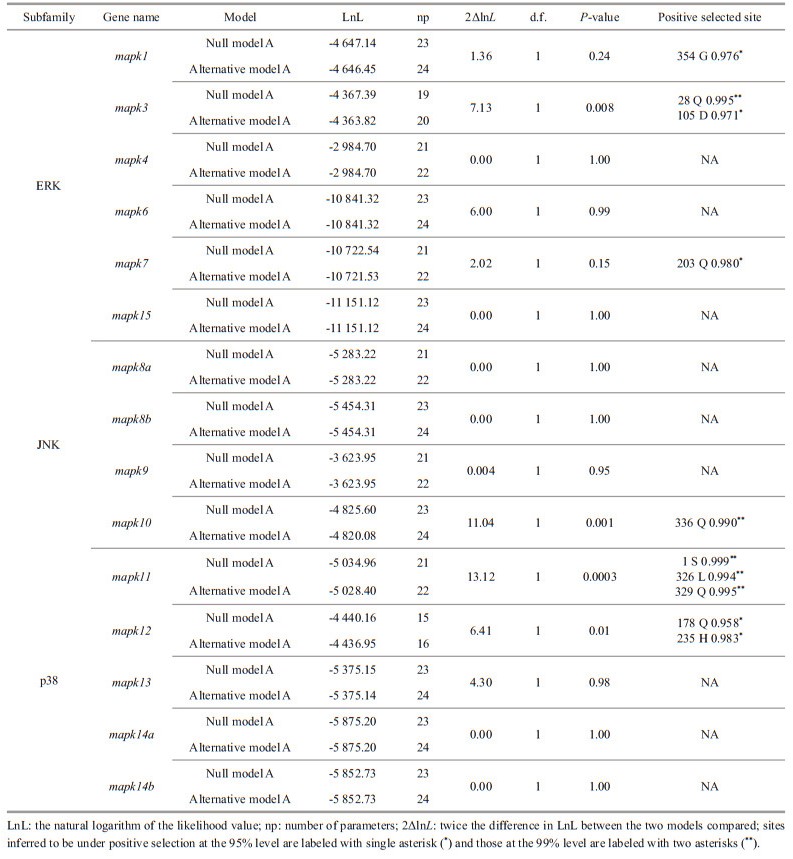
Each mapk gene of the representative species was selected to construct the corresponding phylogenetic tree. The clades of black rockfish were labeled as foreground branches for the estimation of selective pressure using branch-site model (Fig. 6; Supplementary Fig.S1). Results clearly show that alternative hypothesis fit significantly better than null hypothesis in mapk1, mapk3, mapk7, mapk10, mapk11, and mapk12 (Table 2), demonstrating that these mapk genes underwent positive selection in black rockfish. Of these selected genes, the posterior probability of 10 amino acid sites was found greater than 0.95, which underwent significantly positive selection in black rockfish. However, other mapk genes seem to be relatively conserved during the evolution.
|
| Fig.6 Phylogenetic tree of mapk genes for branch-sites model The phylogenetic tree was constructed using software MEGA 7.0 based on the neighbor-joining method and Jones-Taylor-Thornton (JTT) model with 1 000 replicates. The mapk genes of black rockfish are marked red. a–f represent the phylogenetic tree of mapk1, mapk3, mapk7, mapk10, mapk11 and mapk12, respectively. |
|
To explore the expression patterns of mapk genes in black rockfish under normal physiological conditions, qRT-PCR were conducted to determine the expression levels of 15 mapks in 8 different tissues. As shown in Fig. 7, mapk genes displayed distinct expression levels in different tissues. Notably, most mapk genes witnessed relatively high expression levels in brain, especially for mapk8a and mapk13. In skin, mapk1, mapk13, mapk6, and mapk15 genes show higher expression abundance.Among them, mapk1 and mapk15 show high expression levels in muscle, kidney, and spleen. In liver, mapk14a show the highest expression level. Additionally, mapk12 and mapk14b were expressed in intestine mainly, while mapk1, mapk13, and mapk14a in gill kept at higher expression levels.

|
| Fig.7 Expression patterns of mapk genes in healthy tissues of black rockfish The data were measured using qRT-PCR and normalized using EF1A as an internal control. The results are represented as the mean±standard deviation of fold changes relative to genes in kidney tissue. Different letters represented significant differences (P < 0.05). |
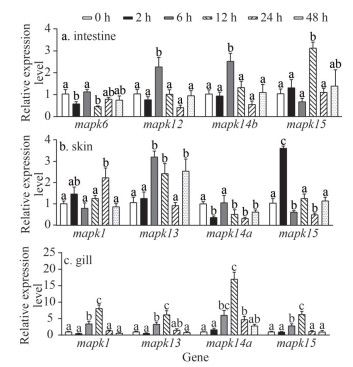
In order to detect the response roles of mapk genes following E. piscicida infection, qRT-PCR was performed to determine the expression patterns of several mapk genes with abundant expression levels in intestine, skin, and gill at different time points (0 h, 2 h, 6 h, 12 h, 24 h, and 48 h) after E. piscicida infection. The results indicate that these selected mapk genes can be induced when black rockfish was infected. In intestine, mapk12, mapk14b, and mapk15 significantly up-regulated at 6 h and 12 h after infection (Fig. 8). Similarly, mapk1 witnessed significant up-regulation at 24 h, and mapk13 significantly up-regulated at 6 h, 12 h, and 48 h after infection in skin. In contrast, the expression level of mapk14a in skin was significantly down-regulated at all detected time points. Dramatic up-regulation of mapk15 in skin was observed at 2 h, while down-regulation appeared at 6 h and 24 h. In gill, mapk1, mapk13, mapk14a, and mapk15 were all significantly up-regulated at 6 h, reached the peak at 24 h and then recovered to normal levels. Notably, mapk14a dramatically increased with more than 17.01 folds at the peak (12 h), demonstrating that it plays an important role in the response to E. piscicida infection in gill of black rockfish. Additionally, mapk15 exhibited diverse expression patterns among intestine, skin, and gill, suggesting that mapk15 may play diverse roles in different mucosal tissues in black rockfish.
|
| Fig.8 Expression patterns of mapk genes in intestine, skin, and gill at different time points after A. salmonicida infection Samples were collected at six time points after A. salmonicida infection, including 0 h, 2 h, 6 h, 12 h, 24 h, and 48 h. Each time points have three biological replicates. Different letters represented significant differences (P < 0.05). |
It is well known that MAPKs play essential roles in various biological processes by regulating related signal pathways (Seger and Krebs, 1995; Westfall et al., 2008; Pathak et al., 2013; Danquah et al., 2014). So far, arduous efforts have been made on the identification of gene families based on genome and transcriptome database in numerous plants and high vertebrates (Cargnello and Roux, 2011; Lu et al., 2015; Wang et al., 2018). However, studies related to mapk gene family remained limited in teleost. This study utilized bioinformatic and experimental approaches to identify mapk genes in black rockfish, investigated their evolutionary relationships, and elucidated their roles in response to pathogens infection.
Totally, 15 mapk genes were systematically identified and functionally characterized in black rockfish, which were further divided into three subfamilies: ERKs, JNKs, and p38s. Similar to previous results of other vertebrates, members of ERK, JNK, and p38 subfamilies in black rockfish possess different dual-phosphorylation sites, including TEY, TPY, and TGY, all are located in the regions of activation loop. The three major clades of the phylogenetic tree are consistent with the classification of subfamilies, suggesting their high conservation in evolution. Moreover, the evolutionary conservation of mapk gene family was further confirmed by the comprehensive analysis of syntenic region, gene structure, protein domain, and chromosomal distribution. The copy numbers of mapk genes in the selected species are relatively conversed and most of them range from 13 to 15. It has been reported that an extra third round of the whole-genome duplications (WGD) occurred in teleost fish, which may lead to duplication or lose some duplicates during this process (Watterson, 1983; Meyer and van de Peer, 2005). Interestingly, the duplicated mapk8 and mapk14 genes were found in teleost only. We thus confirmed that they are teleost-specific, and speculated that whole-genome duplication or recombination events contributed to their duplication, which is supported by previous studies (Vogel et al., 2005; Glasauer and Neuhauss, 2014). To better understand the changes to which these mapk genes have been subjected during evolution, selective pressure analysis was conducted. Totally, 10 positive selection sites were identified in mapk1 (1 site), mapk3 (2 sites), mapk7 (1 site), mapk10 (1 site), mapk11 (3 sites), and mapk12 (2 sites). These genes and sites under positive selection pressure, which may be related to the adaptive evolution of black rockfish. However, further exploration is still needed to clarify the functions of the positively selected amino acid sites in these mapk genes. However, the positively selected amino acid sites in these genes affect or alter their specific biological functions remains to be further explored.
Due to the key roles of mapk genes in almost all aspects of growth, development and response to environment stimuli, the expressions of mapk genes in different development stages and tissues have been well characterized in many species, such as zebrafish (Krens et al., 2006), seahorse (Hippocampus erectus) (Wang et al., 2021), Yesso scallop (Patinopecten yessoensis) (Sun et al., 2016), and Pacific white shrimp (Litopenaeus vannamei) (Yan et al., 2013). In black rockfish, mapk genes displayed different expression patterns in the 8 tested tissues, implying their diverse physiological functions among tissues. Almost all mapk genes were expressed at relatively high levels in brain. The result demonstrated that the mapk genes may be closely associated with the complex patterns of signal transduction in the brain of black rockfish (Kaminska et al., 2009; Zhou et al., 2015). High expressions of mapk1, mapk13, and mapk14a genes were detected in main osmoregulatory tissue of gill, suggesting they may participate in the response to osmotic stresses. Especially, mapk14a is important for the adaptation to hyper-osmotic stress in the gill of spotted sea bass (Tian et al., 2019). In addition, gill along with skin and intestine are the main mucosal tissues of fish, which are the primary sites for host-bacteria interaction and key components of the innate immune system (Gomez et al., 2013; Liu et al., 2016). The abundant expression of these mapk genes in these mucosal tissues reflected the defense mechanisms of innate immunity against bacteria or other pathogens in black rockfish.
Edwardsiella piscicida is a common and important pathogen causing gastrointestinal and systemic infections to aquatic animals, especially fish (Schlenker and Surawicz, 2009; Leung et al., 2012). Similarly, E. piscicida also causes the disease of black rockfish with economic losses. The mapk gene has been shown the ability as regulator of innate and adaptive immune responses. Therefore, the potential roles of mapk genes in immune response of black rockfish were investigated via detecting the expression levels of mapk genes after E. piscicida infection. In this study, the expression levels of several mapk genes in gill, skin, and intestine tissues were significantly changed after E. piscicida infection, suggesting their important roles in mucosal immunity. In detail, the p38 mapk genes, including mapk12 (p38γ), mapk13 (p38δ), mapk14a (p38αa), and mapk14b (p38αb), were all up-regulated at all the time points in the examined tissues, except for mapk14a in skin. Similarly, the expression of p38 mapk genes was also induced by bacterial or pathogen simulation in mucosal tissues of grouper (Epinephelus coioides) (Sun et al., 2017), Chinese shrimp (Fenneropenaeus chinensis) (He et al., 2018), and grass carp (Ctenopharyngodon idella) (Sun et al., 2020). Evidence has been demonstrated that p38 mapk can help host in preventing bacterial infection in aquatic invertebrates and vertebrates (Hansen and Jørgensen, 2007; Uribe et al., 2011; Sun et al., 2020). Moreover, p38 mapk genes can be phosphorylated by transcription factor and translational elements, then inflammatory responses be activated, and can regulate the expressions of numerous pro-inflammatory cytokines such as TNF-α and interleukins (Ils) (Lee et al., 1994; Raingeaud et al., 1995; Zarubin and Han, 2005; Hansen and Jørgensen, 2007; Sun et al., 2019; Zhang et al., 2019). Moreover, we found that E. piscicida infection could significantly induce the expression of mapk1 (erk2) and mapk15 (erk7/8) in mucosal tissues. The results confirmed the function of ERK mapk genes in bacterial-mediated immune response in black rockfish. So far, ERK studies of immune response were mainly focused on mainly erk1 and erk2 genes. The up-regulated expression of erk2 gene was observed in the gill of Chinese shrimp (Li et al., 2013) and grouper (Sun et al., 2018). More importantly, inhibition to erk1/erk2 signaling in zebrafish would diminish the host antibacterial defense and stimulate the pathogenesis of E. piscicida, providing direct evidence that erk1/erk2 signaling can play vital roles in response to E. piscicida infection (Yang et al., 2007). However, the understanding of erk7/8 gene and their biological function in mucosal tissues are still limited.
5 CONCLUSIONTotally, 15 mapk genes were systematically identified in black rockfish, and phylogenetic positions, syntenic analysis were performed to provide additional evidence for their annotations. Ten sites in 6 mapk genes (mapk1, mapk3, mapk7, mapk10, mapk11, and mapk12) were found to undergo significantly positive selection in black rockfish. Moreover, the results of qRT-PCR show that mapk genes displayed distinct expression patterns in different tissues. The expression patterns of mapk12, mapk13, mapk14a, mapk14b, and mapk15 were significantly changed in mucosal tissues after E. piscicida infection, indicating the important roles of mapk genes against bacterial infection in black rockfish. However, further studies are still needed to characterize the immune response mechanism of mapk genes in teleost.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Supplementary material (Supplementary Tables S1–2 and Fig.S1) is available in the online version of this article at https://doi.org/10.1007/s00343-023-2314-3.
Arany I, Megyesi J K, Reusch J E, et al. 2005. CREB mediates ERK-induced survival of mouse renal tubular cells after oxidant stress. Kidney International, 68(4): 1573-1582.
DOI:10.1111/j.1523-1755.2005.00569.x |
Bailey T L, Boden M, Buske F A, et al. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research, 37(S2): W202-W208.
DOI:10.1093/nar/gkp335 |
Cao M, Yan X, Yang N, et al. 2020. Genome-wide characterization of Toll-like receptors in black rockfish Sebastes schlegelii: evolution and response mechanisms following Edwardsiella tarda infection. International Journal of Biological Macromolecules, 164: 949-962.
DOI:10.1016/j.ijbiomac.2020.07.111 |
Cargnello M, Roux P P. 2011. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiology and Molecular Biology Reviews, 75(1): 50-83.
DOI:10.1128/MMBR.00031-10 |
Chen C J, Chen H, Zhang Y, et al. 2020. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Molecular Plant, 13(8): 1194-1202.
DOI:10.1016/j.molp.2020.06.009 |
Cheng Y T, Sun F, Wang L Y, et al. 2020. Virus-induced p38 MAPK activation facilitates viral infection. Theranostics, 10(26): 12223-12240.
DOI:10.7150/thno.50992 |
Cowan K J, Storey K B. 2003. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. Journal of Experimental Biology, 206(7): 1107-1115.
DOI:10.1242/jeb.00220 |
Danquah A, de Zelicourt A, Colcombet J, et al. 2014. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnology Advances, 32(1): 40-52.
DOI:10.1016/j.biotechadv.2013.09.006 |
Dong C, Davis R J, Flavell R A. 2002. MAP kinases in the immune response. Annual Review of Immunology, 20: 55-72.
DOI:10.1038/nri3495 |
Edgar R C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5): 1792-1797.
DOI:10.1093/nar/gkh340 |
Garrington T P, Johnson G L. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Current Opinion in Cell Biology, 11(2): 211-218.
DOI:10.1016/s0955-0674(99)80028-3 |
Glasauer S M K, Neuhauss S C F. 2014. Whole-genome duplication in teleost fishes and its evolutionary consequences. Molecular Genetics and Genomics, 289(6): 1045-1060.
DOI:10.1007/s00438-014-0889-2 |
Gomez D, Sunyer J O, Salinas I. 2013. The mucosal immune system of fish: the evolution of tolerating commensals while fighting pathogens. Fish & Shellfish Immunology, 35(6): 1729-1739.
DOI:10.1016/j.fsi.2013.09.032 |
Hansen T E, Jørgensen J B. 2007. Cloning and characterisation of p38 MAP kinase from Atlantic salmon: a kinase important for regulating salmon TNF-2 and IL-1β expression. Molecular Immunology, 44(12): 3137-3146.
DOI:10.1016/j.molimm.2007.02.006 |
He Y Y, Yao W L, Liu P, et al. 2018. Expression profiles of the p38 MAPK signaling pathway from Chinese shrimp Fenneropenaeus chinensis in response to viral and bacterial infections. Gene, 642: 381-388.
DOI:10.1016/j.gene.2017.11.050 |
Jia Q J, Fan Z J, Yao C L. 2015. Identif. ication and expression profiles of ERK2 and ERK5 in large yellow croaker (Larimichthys crocea) after temperature stress and immune challenge. Fish & Shellfish Immunology, 44(2): 410-419.
DOI:10.1016/j.fsi.2015.03.006 |
Kaminska B, Gozdz A, Zawadzka M, et al. 2009. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. The Anatomical Record, 292(12): 1902-1913.
DOI:10.1002/ar.21047 |
Kitamura S I, Jung S J, Kim W S, et al. 2006. A new genotype of lymphocystivirus, LCDV-RF, from lymphocystis diseased rockfish. Archives of Virology, 151(3): 607-615.
DOI:10.1007/s00705-005-0661-3 |
Krens S F G, He S M, Spaink H P, et al. 2006. Characterization and expression patterns of the MAPK family in zebrafish. Gene Expression Patterns, 6(8): 1019-1026.
DOI:10.1016/j.modgep.2006.04.008 |
Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7): 1870-1874.
DOI:10.1093/molbev/msw054 |
Kyriakis J M, Avruch J. 2012. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiological Reviews, 92(2): 689-737.
DOI:10.1152/physrev.00028.2011 |
Lee J C, Laydon J T, McDonnell P C, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature, 372(6508): 739-746.
DOI:10.1038/372739a0 |
Leung K Y, Siame B A, Tenkink B J, et al. 2012. Edwardsiella tarda—virulence mechanisms of an emerging gastroenteritis pathogen. Microbes and Infection, 14(1): 26-34.
DOI:10.1016/j.micinf.2011.08.005 |
Li X P, Meng X H, Kong J, et al. 2013. Identification, cloning and characterization of an extracellular signal-regulated kinase (ERK) from Chinese shrimp, Fenneropenaeus chinensis. Fish & Shellfish Immunology, 35(6): 1882-1890.
DOI:10.1016/j.fsi.2013.09.021 |
Liu F Q, Su B F, Gao C B, et al. 2016. Identification and expression analysis of TLR2 in mucosal tissues of turbot (Scophthalmus maximus L.) following bacterial challenge. Fish & Shellfish Immunology, 55: 654-661.
DOI:10.1016/j.fsi.2016.06.047 |
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods, 25(4): 402-408.
DOI:10.1006/meth.2001.1262 |
Lu K, Guo W J, Lu J X, et al. 2015. Genome-wide survey and expression profile analysis of the mitogen-activated protein kinase (MAPK) gene family in Brassica rapa. PLoS One, 10(7): e0132051.
DOI:10.1371/journal.pone.0132051 |
Ma L M, Wang W J, Liu C H, et al. 2013. Selection of reference genes for reverse transcription quantitative real-time PCR normalization in black rockfish (Sebastes schlegeli). Marine Genomics, 11: 67-73.
DOI:10.1016/j.margen.2013.08.002 |
Menon M B, Gropengießer J, Fischer J, et al. 2017. p38MAPK/MK2-dependent phosphorylation controls cytotoxic RIPK1 signalling in inflammation and infection. Nature Cell Biology, 19(10): 1248-1259.
DOI:10.1038/ncb3614 |
Meyer A, Van de Peer Y. 2005. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD). Bioessays, 27(9): 937-945.
DOI:10.1002/bies.20293 |
Mulder N, Apweiler R. 2007. InterPro and InterProScan: tools for protein sequence classification and comparison. Methods in molecular biology, 396: 59-70.
DOI:10.1007/978-1-59745-515-2_5 |
Pathak R K, Taj G, Pandey D, et al. 2013. Modeling of the MAPK machinery activation in response to various abiotic and biotic stresses in plants by a system biology approach. Bioinformation, 9(9): 443-449.
DOI:10.6026/97320630009443 |
Raingeaud J, Gupta S, Rogers J S, et al. 1995. Pro-inflammatory Cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. Journal of Biological Chemistry, 270(13): 7420-7426.
|
Roux P P, Blenis J. 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiology and Molecular Biology Reviews, 68(2): 320-344.
DOI:10.1128/MMBR.68.2.320-344.2004 |
Schlenker C, Surawicz C M. 2009. Emerging infections of the gastrointestinal tract. Best Practice & Research Clinical Gastroenterology, 23(1): 89-99.
DOI:10.1016/j.bpg.2008.11.014 |
Seger R, Krebs E G. 1995. The MAPK signaling cascade. The FASEB Journal, 9(9): 726-735.
DOI:10.1016/B978-0-12-394447-4.30014-1 |
Sun H Y, Huang M Z, Li Y W, et al. 2017. Two novel p38 MAPKs identified from Epinephelus coioides and their expression pattern in response to Cryptocaryon irritans infection. Fish & Shellfish Immunology, 67: 459-466.
DOI:10.1016/j.fsi.2017.06.004 |
Sun H Y, Huang M Z, Mo Z Q, et al. 2018. Characterization and expression patterns of ERK1 and ERK2 from Epinephelus coioides against Cryptocaryon irritans infection. Fish & Shellfish Immunology, 74: 393-400.
DOI:10.1016/j.fsi.2017.12.050 |
Sun J J, Wang L L, Wu Z J, et al. 2019. P38 is involved in immune response by regulating inflammatory cytokine expressions in the Pacific oyster Crassostrea gigas. Developmental & Comparative Immunology, 91: 108-114.
DOI:10.1016/j.dci.2018.10.011 |
Sun Y, Liu W Z, Liu T, et al. 2015. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. Journal of Receptors and Signal Transduction, 35(6): 600-604.
DOI:10.3109/10799893.2015.1030412 |
Sun Y, Zhang L L, Zhang M M, et al. 2016. Characterization of three mitogen-activated protein kinases (MAPK) genes reveals involvement of ERK and JNK, not p38 in defense against bacterial infection in Yesso scallop Patinopecten yessoensis. Fish & Shellfish Immunology, 54: 507-515.
DOI:10.1016/j.fsi.2016.04.139 |
Sun Y D, Xu W Q, Li D, et al. 2020. p38 mitogen-activated protein kinases (MAPKs) are involved in intestinal immune response to bacterial muramyl dipeptide challenge in Ctenopharyngodon idella. Molecular Immunology, 118: 79-90.
DOI:10.1016/j.molimm.2019.12.007 |
Tian Y, Wen H S, Qi X, et al. 2019. Identification of mapk gene family in Lateolabrax maculatus and their expression profiles in response to hypoxia and salinity challenges. Gene, 684: 20-29.
DOI:10.1016/j.gene.2018.10.033 |
Umasuthan N, Bathige S D N K, Noh J K, et al. 2015. Gene structure, molecular characterization and transcriptional expression of two p38 isoforms (MAPK11 and MAPK14) from rock bream (Oplegnathus fasciatus). Fish & Shellfish Immunology, 47(1): 331-343.
DOI:10.1016/j.fsi.2015.09.018 |
Uribe C, Folch H, Enríquez R, et al. 2011. Innate and adaptive immunity in teleost fish: a review. Veterinární Medicína, 56(10): 486-503.
DOI:10.17221/3294-VETMED |
Vogel C, Teichmann S A, Pereira-Leal J. 2005. The relationship between domain duplication and recombination. Journal of Molecular Biology, 346(1): 355-365.
DOI:10.1016/j.jmb.2004.11.050 |
Wang G, Wang T, Jia Z H, et al. 2018. Genome-wide bioinformatics analysis of MAPK gene family in kiwifruit (Actinidia chinensis). International Journal of Molecular Sciences, 19(9): 2510.
DOI:10.3390/ijms19092510 |
Wang K, Wang X, Zou Q, et al. 2021. Genome-wide evolution of MAPKs family and their expression in response to bacterial infection in seahorse Hippocampus erectus. Journal of Oceanology and Limnology, 39(6): 2309-2321.
DOI:10.1007/s00343-020-0332-y |
Watterson G A. 1983. On the time for gene silencing at duplicate loci. Genetics, 105(3): 745-766.
DOI:10.1093/genetics/105.3.745 |
Wei X M, Zhang Y, Li C, et al. 2020. The evolutionarily conserved MAPK/Erk signaling promotes ancestral T-cell immunity in fish via c-Myc-mediated glycolysis. Journal of Biological Chemistry, 295(10): 3000-3016.
DOI:10.1074/jbc.RA119.012231 |
Westfall P J, Patterson J C, Chen R E, et al. 2008. Stress resistance and signal fidelity independent of nuclear MAPK function. Proceedings of the National Academy of Sciences of the United States of America, 105(34): 12212-12217.
DOI:10.1073/pnas.0805797105 |
Yan H, Zhang S, Li C Z, et al. 2013. Molecular characterization and function of a p38 MAPK gene from Litopenaeus vannamei. Fish & Shellfish Immunology, 34(6): 1421-1431.
DOI:10.1016/j.fsi.2013.02.030 |
Yang Z H. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution, 24(7): 1586-1591.
DOI:10.1093/molbev/msm088 |
Zarubin T, Han J H. 2005. Activation and signaling of the p38 MAP kinase pathway. Cell Research, 15(1): 11-18.
DOI:10.1038/sj.cr.7290257 |
Zhang C N, Rahimnejad S, Lu K L, et al. 2019. Molecular characterization of p38 MAPK from blunt snout bream (Megalobrama amblycephala) and its expression after ammonia stress, and lipopolysaccharide and bacterial challenge. Fish & Shellfish Immunology, 84: 848-856.
DOI:10.1016/j.fsi.2018.10.074 |
Zhou J, Du T, Li B M, et al. 2015. Crosstalk between MAPK/ERK and PI3K/AKT signal pathways during brain ischemia/reperfusion. ASN Neuro, 7(5): 1759091415602.
DOI:10.1177/1759091415602463 |
Zhu J, Cai L, Zhang T H, et al. 2014. Identification and characterization of a p38-like gene from amphioxus (Branchiostoma belcheri): an insight into amphioxus innate immunity and evolution. Fish & Shellfish Immunology, 41(2): 421-427.
DOI:10.1016/j.fsi.2014.09.028 |
 2023, Vol. 41
2023, Vol. 41



