Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZOU Desheng, CAO Weian, LIU Guilong, NING Junhao, LU Xia, WANG Jinjing, CHEN Min, LIU Bo, ZHANG Jinsheng, WANG Chunde
- Effects of acute and chronic thermal stress on survival, apoptosis, and transcriptional responses of Scapharca broughtonii
- Journal of Oceanology and Limnology, 41(6): 2363-2373
- http://dx.doi.org/10.1007/s00343-022-2092-3
Article History
- Received Mar. 11, 2022
- accepted in principle Jun. 16, 2022
- accepted for publication Oct. 13, 2022
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Yantai Spring-Sea AquaSeed Co., Ltd., Yantai 264006, China;
4 Qingdao Agricultural University, Qingdao 266109, China;
5 Zhaoyuan Xiadian Agricultural Technology Promotion Station, Yantai 265415, China
As a result of global warming, the average surface temperature of the global sea has risen by almost 1 ℃ in the last 40 years. Simulations by the Intergovernmental Panel on Climate Change (IPCC) stated that global average sea surface temperatures could rise as much as 4.8 ℃ by the end of the 21st century if these trends continue (IPCC, 2013). Extreme ocean warming events in the summers of three consecutive years from 2016 to 2018 were reported in the East China Sea and South Yellow Sea (Gao et al., 2020). Marine organisms inhabiting nearshore areas, unlike terrestrial animals, are usually unable to effectively evade from high-temperature environments (Burrows et al., 2019).
Temperature is a prominent environmental stress to marine organisms, especially intertidal marine molluscs, whose survival, growth, development, reproduction, metabolism, immunity, and geographical distribution can be seriously affected. Proper temperature regulation is beneficial to the growth, development, and reproduction of intertidal organisms, as well demonstrated in the snail Plicopurpura pansa (Naegel and López-Rocha, 2007). However, if the threshold temperature is exceeded, it will adversely affect many aspects of life. It has been reported that fluctuations in temperature greatly affected the survival rate of Crepidula fornicata in Nahant, Massachusetts (Pechenik et al., 2020). To a certain extent, thermal stress tends to cause physiological imbalance in animals, directly stimulating the metabolism of the body and leading to excessive accumulation of reactive oxygen species (ROS) (Lesser, 2006). Consequently, key cellular components (e.g., proteins, DNA, and membrane lipids of the body) are damaged, leading to the disruption of the physiological performance and gene expression in marine organisms (Li et al., 2020; Clark et al., 2021), which triggers a series of cellular defense events (Meng et al., 2014). Studies have shown that temperature change adversely induced body damage by activating the enzyme defense system, antioxidant system, immune system, and apoptotic pathway in marine organisms (Zhou et al., 2019; Ning et al., 2021). Molluscs inhabiting in intertidal waters are often exposed to acute or chronic thermal stresses, and the adaptability of intertidal organisms to climate warming is poor (Stillman, 2003). Therefore, studying the biological responses of these intertidal molluscs to thermal stress may provide insights into the mechanism of adaptation of these intertidal molluscs to high temperatures.
The ark shell, Scapharca broughtonii, is a large intertidal marine mollusc widely distributed in the Sea of Japan, Bohai Sea, Yellow Sea, and East China Sea. It is highly demanded in the market due to its delicious meat, high protein, and low fat (Wang et al., 2013). In recent years, due to overfishing and habitat destruction, the natural resources of S. broughtonii have been dramatically declining. With the global warming, rising of water temperature is also becoming one of the most important factors threatening their survival, especially in hot summers (Ning et al., 2021). Therefore, exploring the apoptosis, physiological properties, and gene expression of S. broughtonii under different thermal stresses will help to comprehensively understand the thermal tolerance of ark shells and provide a scientific basis for the selection of new heat-resistant strains.
2 MATERIAL AND METHOD 2.1 Experimental animalHealthy S. broughtonii (34.30±3.09 mm in shell length, 14.58±4.14 g in whole weight) were obtained from a seafood market in Yantai, China (37°51′N, 121°46′E). The ark shells were acclimated in 200-L tanks with filtered seawater (salinity 29±0.5, pH 8.13±0.2) at 14±0.5 ℃ for one week prior to experimentation. The animals were fed daily with Chaetoceros muelleri and the seawater was changed twice a day during this period.
2.2 Determination of the upper median lethal temperatureAfter acclimation, 480 healthy animals were randomly divided into eight groups, a control group at 14 ℃ and seven experimental groups at 17, 20, 23, 26, 29, 32, and 35 ℃, respectively, with 3 replicates in each group. The water in each group was heated to the designated temperature at a rate of 1 ℃/h by a heating rod and maintained at that temperature for 96 h. The ark shells were checked once every 12 h and those did not respond to mechanical touch at the mantle were deemed to dead and selected out. The seawater in the tanks was replaced by clean seawater heated to the same temperature in advance, twice a day. Determination of median lethal temperature (LT50) within 96 h by two-point method (Liu et al., 2007) (Fig. 1).
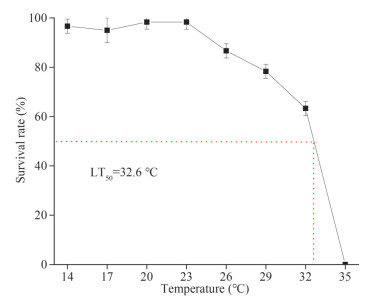
|
| Fig.1 Determination of 96-h LT50 Sixty healthy animals were exposed to 14, 17, 20, 23, 26, 29, 32, and 35 ℃ for 96 h (n=3). |
During the experiment, 1 200 animals in healthy condition were randomly chosen and divided equally into four groups, including the acute heating control group (AHCG), the acute heating experimental group (AHEG), the chronic heating control group (CHCG), and the chronic heating experimental group (CHEG). Three replicates were set for each group. The ark shells were checked once every 12 h and those did not respond to mechanical touch at the mantle were deemed to dead and selected out during the experiment.
In the AHEG group, seawater temperature was heated from 14 ℃ to 33 ℃ at a rate of 1 ℃/h. After the water temperature reached 33 ℃, nine animals were randomly selected for sampling at 1, 3, 6, 12, and 24 h (Fig. 2a). In the AHCG group, ark shells were cultured at room temperature (14±0.5 ℃) and nine animals were randomly collected at 1, 6, and 24 h. Animals were not fed during the experiment.
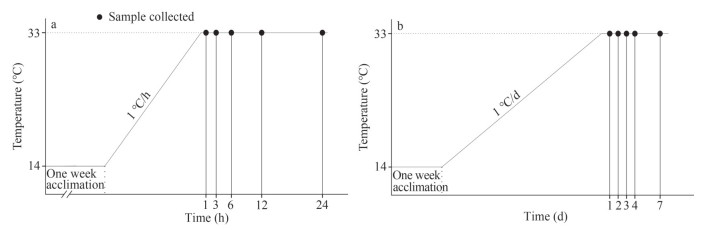
|
| Fig.2 Schematic outlines of the acute (a) and chronic (b) heating treatment experiment |
In the CHEG group, seawater temperature was heated slowly from 14 ℃ to 33 ℃ at a rate of 1 ℃/d, and nine animals were randomly sampled on Days 1, 2, 3, 4, and 7 after the water temperature reached 33 ℃ (Fig. 2b). In the CHCG group, the animals were cultured at room temperature (14±0.5 ℃) and nine animals were randomly sampled on Days 1, 3, and 7. During the experiments, animals were fed with concentrated microalgae diet twice a day, and the seawater was changed using preheated seawater 2 h after each feeding.
The sampled ark shells were dissected to collect their hemocytes and gills immediately after sampling. From each animal, 0.5 mL of hemolymph was collected using a sterile syringe filled with 0.5 mL of precooled anticoagulant buffer (336-mmol/L NaCl, 27-mmol/L sodium citrate, 9-mmol/L EDTA-Na2, 115-mmol/L glucose, pH 7.0). The hemolymphs collected from per animal at the same time point were pooled together and filtered with a 300-mesh sieve. The hemocytes centrifugated at 1 000×g at 4 ℃ for 5 min were used for measurement of apoptosis rate and necrosis rate immediately. Meanwhile, the gills from 3 animals of the same time point were pooled together, thoroughly washed with phosphate-buffered saline (PBS, pH 7.4), and stored at -80 ℃ for further analyses.
2.4 Apoptosis and necrosis rate of hemocytesThe hemocyte pellets were washed twice with PBS (pH 7.4), resuspended in 195-µL 1×binding buffer solution buffer, and stained with 10-µL PI dyes and 5-μL annexin V-FITC at 20 ℃ in dark for 20 min. Analyses of cell suspensions by the flow cytometry (CytoFLEX, USA). The apoptosis rate was expressed as the percentage of the number of PI negative and V-FITC positive cells in the total number of cells. The necrosis rate was represented as the percentage of the number of PI positive and V-FITC positive cells in the total number of cells (Schutte et al., 1998).
2.5 RNA extraction and cDNA synthesisTotal RNA was extracted from the gill samples by TRIzol reagent (Invitrogen, USA), genomic DNA was digested with RNase-free DNase I (Vazyme, China). The purity and concentration of the RNA samples were analyzed using Nanodrop 2000 device (Thermo, USA), and RNA integrity was determined by 1% agarose gel electrophoresis. Synthesis of cDNA with the HiScript® Ⅲ RT Super Mix for qPCR Kit (Vazyme, China) and stored at -20 ℃ until further analyses.
2.6 Gene expression analysisThe expression levels of twelve genes, including heat shock proteins (HSP90 and HSP20), apoptosis-related genes (TRAF6, GRP78, NIX, and Casp-3), antioxidative-related genes (GST and MRP) and immune-related genes (HbⅡB, NOS-1, HO-1, and ENO-1), were selected to verify their responses to thermal stresses. Primers used for the qRT-PCR analyses are listed in Table 1. 18S rRNA was used as the internal reference for the normalization of gene expression. The reactions were performed on the QuantStudioTM 5 Real-Time PCR Instrument (Applied Biosystems, USA), and fluorescent dye was SYBR green Ⅱ. PCR amplifications were performed using a 10-μL reaction mixture containing 0.2 μL of each primer (10 μmol/L), 1 μL of the template cDNA, 3.6-μL ddH2O and 5 μL of 2×Taq Pro Universal SYBR qPCR Master Mix (Vazyme, China). The PCR program was set as follows: 95 ℃ for 60 s, followed by 40 cycles of 95 ℃ for 5 s and 60 ℃ for 15 s. The amplification specificity was confirmed by a melting curve analyses. All reactions were performed in triplicates (Ning et al., 2021). The relative expression of the selected genes was analyzed with the 2-ΔΔCt method (Livak and Schmittgen, 2001).
SPSS 17.0 software was used for statistical analyses. Significant differences were analyzed using one-way analyses of variance and Duncan's test. The statistically significant differences were considered as P < 0.05 (*) and P < 0.01 (**).
3 RESULT 3.1 Determination of 96-h LT50In this study, the upper LT50 of S. broughtonii within 96 h was determined to be 32.6 ℃ (Fig. 1).
3.2 The cumulative mortality of ark shells during acute and chronic heating treatmentIn the AHEG, the cumulative mortality of ark shells increased linearly with the extension of stress time and reached 55.7% within 96 h. However, in the CHEG, the cumulative mortality was much lower than that in the AHEG, with a cumulative mortality rate of only 21.67% within 96 h. There were almost no deaths during the experiment in the AHCG and CHCG (Fig. 3).
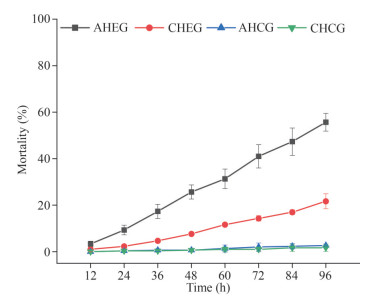
|
| Fig.3 Cumulative mortality of ark shells during the acute and chronic heating experiment Three replicates were set for each group. AHCG: acute heating control group; AHEG: acute heating experimental group; CHCG: chronic heating control group; CHEG: chronic heating experimental group. |
In the AHEG, the apoptosis rate of ark shell hemocytes was increased significantly and reached the peak value (18.97%) at 3 h, then decreased slowly and restored to the initial level at 24 h (Fig. 4a). However, the necrosis rate of hemocytes showed a linear increase with time and reached the maximum value (11.86%) at 24 h (Fig. 4b). However, in the CHEG, the apoptosis and necrosis rate of hemocytes showed a similar change trend: increase significantly and reach the maximum value on Day 1, then a linear decrease from Days 1 to 4 followed by a slight increase on Day 7. Moreover, the apoptosis and necrosis rates of hemocytes in the CHEG group were higher than those in the control group (Fig. 4c–d).
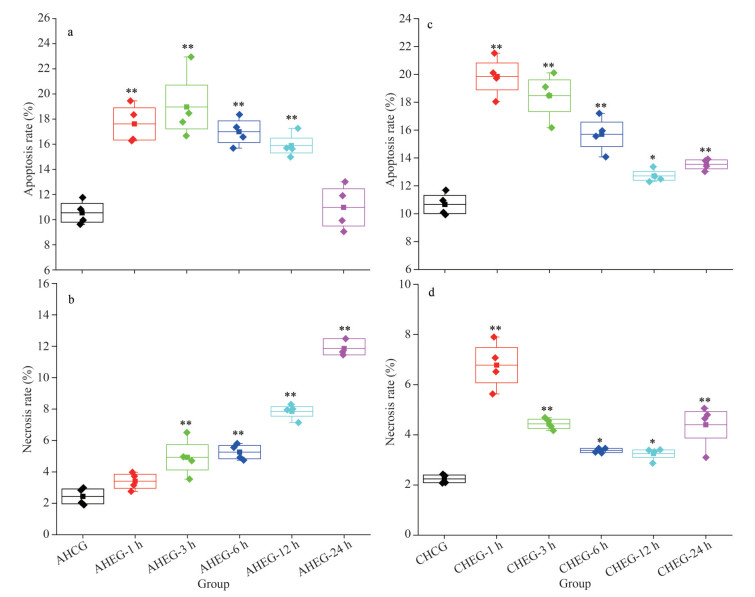
|
| Fig.4 Apoptosis and necrosis rates of ark shell hemocytes under acute and chronic heating stress AHCG: acute heating control group; AHEG: acute heating experimental group; CHCG: chronic heating control group; CHEG: chronic heating experimental group. Statistically significant differences from control are labeled with * (P < 0.05) and ** (P < 0.01). |
During the acute heating treatment, the expressions of heat shock protein genes (HSP20 and HSP90), apoptosis-related genes (TRAF6, GRP78, NIX, and Casp-3), antioxidative-related genes (GST and MRP) and immune-related genes (HbⅡB, NOS-1, HO-1, and ENO-1) were up-regulated significantly under acute thermal stress, and the peak values were mostly observed at 1 and 12 h. However, the expressions of HSP90 and Casp-3 were down-regulated manifestly at 24 h (Fig. 5).
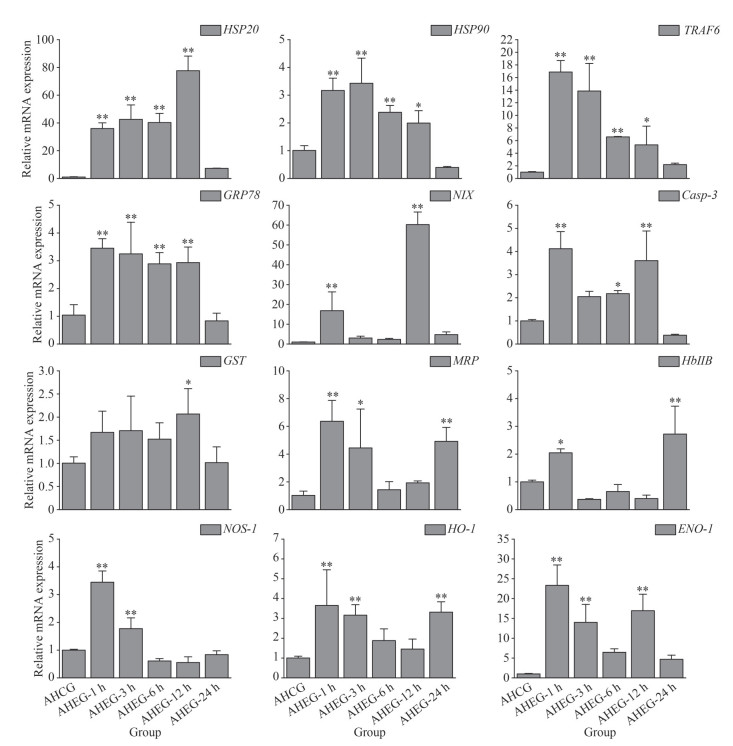
|
| Fig.5 Transcriptional response of ark shell gills to acute thermal stress HSP: heat shock protein; TRAF6: TNF receptor-associated factor 6; GRP78: 78-kDa glucose-regulated protein; NIX: nip3-like protein X; Casp-3: caspase-3; GST: glutathione S-transferase; MRP: multidrug resistance protein; HbⅡB: hemoglobin ⅡB; NOS: nitric oxide synthases-1; HO-1: heme oxygenase-1; ENO-1: enolase-1. Statistically significant differences from control are labeled with * (P < 0.05) and ** (P < 0.01). |
During the chronic heating treatment, the expression levels of the twelve selected genes were up-regulated significantly, with the maximum values mainly on Days 3 and 4. However, the expression of MRP was down-regulated significantly on Day 1 (Fig. 6).
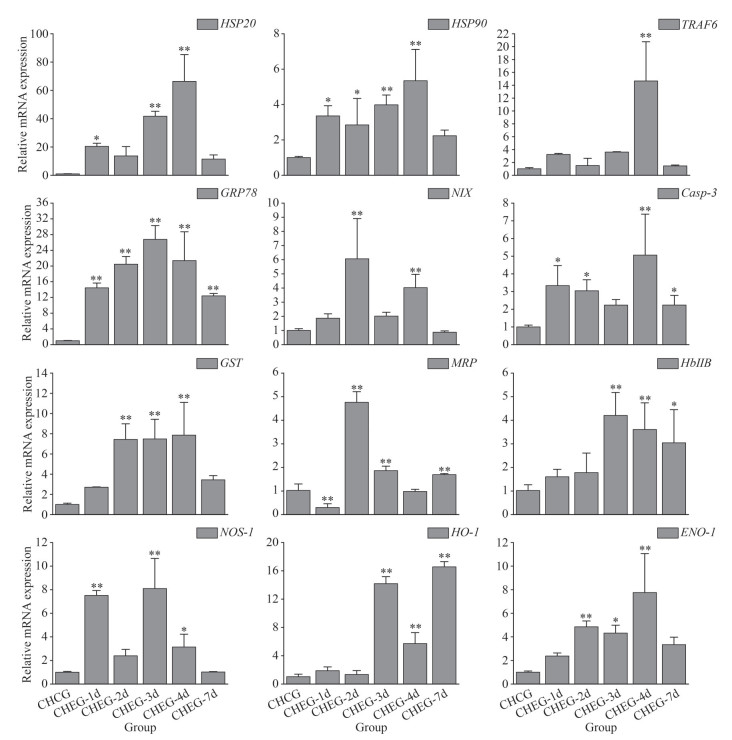
|
| Fig.6 Transcriptional response of ark shell gills to chronic thermal stress Statistically significant differences from control are labeled with * (P < 0.05) and ** (P < 0.01). |
In recent years, ocean warming has led to an obvious decline in marine fisheries productivity and sustainable fishing potential, and overfishing has reduced the adaptive capacity of many marine invertebrates to a warmer ocean (Free et al., 2019), which may have contributed to the frequent occurrence of invertebrate deaths. Therefore, it is necessary to investigate the physiological responses and adaptive mechanism of marine invertebrates, especially animals with poor mobility, to thermal stresses. In this study, the upper LT50 of S. broughtonii within 96 h much lower than the maximum temperature in the north inshore of China, especially the intertidal flats where ark shells normally dwell (Ning et al., 2015). Obviously, the ark shells may be subjected to severe heat stresses in hot summers.
It has been confirmed that apoptosis and necrosis are important physiological processes of organisms in response to environmental stress, which could be induced significantly by thermal stress (Cheng et al., 2018; Zhou et al., 2019; Zou et al., 2021). Similarly, in this study, the apoptosis and necrosis rates of hemocytes were also increased significantly when ark shells were subjected to thermal stress. Under acute heat stress, the apoptosis rate remained at a high level and the necrosis rate increased in a time-dependent manner, indicating that the apoptosis and necrosis pathways of ark shells has been activated and the stress injury might be irreparable with the prolongation of stress duration. This result is consistent with previously published information in Crassostrea gigas (Yang et al., 2017) and S. subcrenata (Zou et al., 2021). However, the difference was that the apoptosis rate and the expression of some related genes in AHEG can return to their original state at 24 h. Related results have also been reported in Patinopecten yessoensis, Litopenaeus vannamei, and Hippopotamus, suggesting that the internal environment of these animals has been disturbed (Jiang et al., 2016; Wang et al., 2019; Pan et al., 2022). Moreover, the significant up-regulation of apoptosis-related genes was also in support of the above viewpoint. However, under chronic thermal stress, the apoptosis and necrosis rates of hemocytes showed a similar trend, a significant increase followed by a slow decline, indicating ark shells might gradually build the adaptability to a suboptimal temperature. The manifestly lower mortality of ark shells under chronic thermal stress compared with that under acute thermal stress also supported the above viewpoint. It is speculated that local adaptation and phenotypic plasticity play crucial roles in the modification of thermal tolerance of organisms (Sanford and Kelly, 2011; Yampolsky et al., 2014).
Heat shock proteins (HSPs) are a family of highly conserved proteins that play key roles in rapid responses of organisms to environmental changes, and the expression of HSPs is often considered as an important indicator to analyze the responses of organisms to temperature stress (Feder and Hofmann, 1999; Srivastava, 2002). Among HSPs, HSP90s are essential chaperone proteins in response to thermal stress in many marine molluscs (Kim et al., 2009; Lim et al., 2016). HSP20s, also named stress induced protein 1, could prevent aggregation of stress-induced proteins and facilitate regeneration of denatured proteins when animals suffer thermal stress (Nover and Scharf, 1997; Ning et al., 2021). In this research, the expression of HSPs (HSP20 and HSP90) was rose remarkably under both acute and chronic heat stresses, which was consistent with our previous results (Ning et al., 2021; Zou et al., 2021). It is suggested that the significant up-regulation of HSPs would help to strengthen the high temperature tolerance of ark shells. Similar results were also reported in Pacific oyster C. gigas (Lim et al., 2016) and pufferfish Takifugu obscurus (Cheng et al., 2018).
Apoptosis is a key physiological protective mechanism of organisms in response to environmental stresses (Cheng et al., 2018; Zhou et al., 2019). Caspase 3 (Casp-3) is responsible for the cleavage of structural proteins and regulatory proteins participating in apoptosis, which ensures the normal progression of apoptosis (Qin et al., 2020). Nip3-like protein X (NIX) is a key member of the BNIP 3 subfamily, which promotes either apoptotic or non-apoptotic cell death (Chinnadurai et al., 2008). Moreover, the anti-apoptotic pathways in molluscs could be activated to counteract the injuries caused by various stresses (Zhang et al., 2012). For instance, Glucose regulated protein 78 kD (GRP78) could reduce the damage caused by apoptosis by inhibiting the activation of Casp-7 (Reddy et al., 2003). Tumor necrosis factor receptor-associated factor 6 (TRAF6) could inhibit the apoptosis of oyster hemocytes through activation of Pellino (Lin et al., 2020). The up-regulation of apoptosis-related genes (Casp-3, NI X, GRP78, and TRAF6) indicated that the apoptotic and anti-apoptotic systems existed in ark shells and may work simultaneously under heat stress, which is consistent with the previous studies reported in S. subcrenata (Ning et al., 2021; Zou et al., 2021).
As poikilothermal animals, molluscan body temperature changes with the ambient temperature, and thus inevitably affects their metabolism and physiological processes (Konstantinov et al., 2003; Brucet et al., 2012). For instance, reactive oxygen species (ROS) can be produced by respiratory burst of phagocytes under thermal stresses, resulting in severe injury to organisms (Bachère et al., 1995; De La Fuente and Victor, 2000). Moreover, elevated seawater temperature might promote the decomposition of ark shells secretions into toxic substances (Eriksson et al., 2013). It has been reported that glutathione S-transferase (GST) and multidrug resistance protein (MRP) are involved in antioxidant defense and cell detoxification (Hayes and Pulford, 1995; Mejdoub et al., 2017; Ning et al., 2021). HbⅡB and Heme oxygenase-1 (HO-1), as the major components of haemoglobin, play important roles in cell detoxification (Geuken et al., 2005; Bao et al., 2013). The significant up-regulation of these genes was observed in this study, suggesting antioxidant defense system provides vital protection for ark shells from irreversible injury caused by thermal stresses. This is consistent with the conclusion found in Dicentrarchus labrax (Hachfi et al., 2012) and S. subcrenata (Zou et al., 2021). Nitric oxide synthases (NOS) are responsible for catalytic production of nitric oxide in mitochondria, which is a vital biological process for maintaining cell metabolism (Haynes et al., 2004). NOS is involved in a variety of functions in invertebrates, and upon stimulation, the expression levels of NOS in midintestine, hepatopancreas, and hemocytes were significantly increased (Li et al., 2012). In the present study, the expression of NOS-1 showed a similar trend, a significant increase followed by a quick decline, under both acute and chronic thermal stress. Our results also showed that the expression of Enolase-1 (ENO-1) was also up-regulated by thermal stress. ENO-1 is an enzyme of the glycolytic pathway that interacts with plasminogen and suggests ENO-1 as a candidate marker for monitoring health status in teleosts (Santana et al., 2021). The significant up-regulation of α-ENO in kidney and spleen of the rainbow trout was also found when challenged with Yersinia ruckeri (Kumar et al., 2018). Therefore, we speculate that ENO-1 can also be used as an indicator of invertebrate response to high temperature stimulation.
5 CONCLUSIONIn summary, the upper LT50 of adult ark shells was determined to be 32.6 ℃. The higher mortality in ark shells under acute thermal stress suggested they suffered more injuries and gradually turned irreversible under acute thermal stress. The apoptosis and necrosis rates of ark shell hemocytes were increased significantly under both acute and chronic thermal stresses, and significant up-regulation of apoptosis-related genes was observed herein, suggesting the apoptotic and anti-apoptotic systems were activated in response to thermal stresses. Moreover, the expression of the genes involved in antioxidant defense, cellular detoxification and homeostasis was increased significantly to protect ark shells from oxidative damage. These results may provide new clues for management strategy of ark shell husbandry and further understanding of the tolerance to thermal stresses in ark shells.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTWe are grateful to all the laboratory members and anonymous reviewers for their technical advice and helpful discussions.
Bachère E, Mialhe E, Noël D, et al. 1995. Knowledge and research prospects in marine mollusc and crustacean immunology. Aquaculture, 132(1-2): 17-32.
DOI:10.1016/0044-8486(94)00389-6 |
Bao Y B, Wang Q, Guo X M, et al. 2013. Structure and immune expression analysis of hemoglobin genes from the blood clam Tegillarca granosa. Genetics and Molecular Research, 12(3): 3110-3123.
DOI:10.4238/2013.February.28.5 |
Brucet S, Boix D, Nathansen L W, et al. 2012. Effects of temperature, salinity and fish in structuring the macroinvertebrate community in shallow lakes: implications for effects of climate change. PLoS One, 7(2): e30877.
DOI:10.1371/journal.pone.0030877 |
Burrows M T, Bates A E, Costello M J, et al. 2019. Ocean community warming responses explained by thermal affinities and temperature gradients. Nature Climate Change, 9(12): 959-963.
DOI:10.1038/s41558-019-0631-5 |
Cheng C H, Guo Z X, Luo S W, et al. 2018. Effects of high temperature on biochemical parameters, oxidative stress, DNA damage and apoptosis of pufferfish (Takifugu obscurus). Ecotoxicology and Environmental Safety, 150: 190-198.
DOI:10.1016/j.ecoenv.2017.12.045 |
Chinnadurai G, Vijayalingam S, Gibson S B. 2008. BNIP3 subfamily BH3-only proteins: mitochondrial stress sensors in normal and pathological functions. Oncogene, 27 Suppl 1(S1): S114-S127.
DOI:10.1038/onc.2009.49 |
Clark M S, Peck L S, Thyrring J. 2021. Resilience in Greenland intertidal Mytilus: the hidden stress defense. Science of the Total Environment, 767: 144366.
DOI:10.1016/j.scitotenv.2020.144366 |
De La Fuente M, Victor V. 2000. Anti-oxidants as modulators of immune function. Immunology & Cell Biology, 78(1): 49-54.
DOI:10.1046/j.1440-1711.2000.00884.x |
Eriksson S P, Hernroth B, Baden S P. 2013. Stress biology and immunology in Nephrops norvegicus. Advances in Marine Biology, 64: 149-200.
DOI:10.1016/B978-0-12-410466-2.00005-4 |
Feder M E, Hofmann G E. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annual Review of Physiology, 61: 243-282.
DOI:10.1146/annurev.physiol.61.1.243 |
Free C M, Thorson J T, Pinsky M L, et al. 2019. Impacts of historical warming on marine fisheries production. Science, 363(6430): 979-983.
DOI:10.1126/science.aau1758 |
Gao G D, Marin M, Feng M, et al. 2020. Drivers of marine heatwaves in the East China Sea and the South Yellow Sea in three consecutive summers during 2016-2018. Journal of Geophysical Research: Oceans, 125(8): e2020JC016518.
DOI:10.1029/2020JC016518 |
Geuken E, Buis C I, Visser D S, et al. 2005. Expression of heme oxygenase-1 in human livers before transplantation correlates with graft injury and function after transplantation. American Journal of Transplantation, 5(8): 1875-1885.
DOI:10.1111/j.1600-6143.2005.00960.x |
Hachfi L, Simide R, Richard S, et al. 2012. Effect of water temperature increase on HO-1 expression in European sea bass (Dicentrarchus labrax L.) tissues. Cellular and Molecular Biology, (Suppl 58): OL1752-6.
DOI:10.1170/205 |
Hayes J D, Pulford D J. 1995. The glutathione S-transferase supergene family: regulation of GST and the contribution of the lsoenzymes to cancer chemoprotection and drug resistance Part I. Critical Reviews in Biochemistry and Molecular Biology, 30(6): 445-520.
DOI:10.3109/10409239509083491 |
Haynes V, Elfering S, Traaseth N, et al. 2004. Mitochondrial nitric-oxide synthase: enzyme expression, characterization, and regulation. Journal of Bioenergetics and Biomembranes, 36(4): 341-346.
DOI:10.1023/B:JOBB.0000041765.27145.08 |
Hoegh-Guldberg O, Mumby P J, Hooten A J, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science, 318(5857): 1737-1742.
DOI:10.1126/science.1152509 |
IPCC. 2013. Climate change 2013: the physical science basis. In: Stocker T F, Qin D, Plattner G K et al. eds. Contribution of Working Group Ⅰ to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge. p. 1535.
|
Jiang W W, Li J Q, Gao Y P, et al. 2016. Effects of temperature change on physiological and biochemical responses of Yesso scallop, Patinopecten yessoensis. Aquaculture, 451: 463-472.
DOI:10.1016/j.aquaculture.2015.10.012 |
Kim M, Ahn I Y, Kim H, et al. 2009. Molecular characterization and induction of heat shock protein 90 in the Antarctic bivalve Laternula elliptica. Cell Stress and Chaperones, 14(4): 363-370.
DOI:10.1007/s12192-008-0090-9 |
Konstantinov A S, Pushkar' V Y, Aver'yanova O V. 2003. Effects of fluctuations of abiotic factors on the metabolism of some hydrobionts. Biology Bulletin of the Russian Academy of Sciences, 30(6): 610-616.
DOI:10.1023/B:BIBU.0000007719.82974.44 |
Kumar G, Hummel K, Noebauer K, et al. 2018. Proteome analysis reveals a role of rainbow trout lymphoid organs during Yersinia ruckeri infection process. Scientific Reports, 8(1): 13998.
DOI:10.1038/s41598-018-31982-6 |
Lesser M P. 2006. Oxidative stress in marine environments: biochemistry and physiological ecology. Annual Review of Physiology, 68: 253-278.
DOI:10.1146/annurev.physiol.68.040104.110001 |
Li S K, Zhang Z, Li C B, et al. 2012. Molecular cloning and expression profiles of nitric oxide synthase (NOS) in mud crab Scylla paramamosain. Fish & Shellfish Immunology, 32(4): 503-512.
DOI:10.1016/j.fsi.2011.12.002 |
Li Y F, Yang X Y, Cheng Z Y, et al. 2020. Near-future levels of ocean temperature weaken the byssus production and performance of the mussel Mytilus coruscus. Science of the Total Environment, 733: 139347.
DOI:10.1016/j.scitotenv.2020.139347 |
Lim H J, Kim B M, Hwang I J, et al. 2016. Thermal stress induces a distinct transcriptome profile in the Pacific oyster Crassostrea gigas. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 19: 62-70.
DOI:10.1016/j.cbd.2016.06.006 |
Lin Y, Mao F, Zhang X Y, et al. 2020. TRAF6 suppresses the apoptosis of hemocytes by activating pellino in Crassostrea hongkongensis. Developmental & Comparative Immunology, 103: 103501.
DOI:10.1016/j.dci.2019.103501 |
Liu Z G, Wang H, Li Z M, et al. 2007. Upper incipient lethal temperature of Argopecten irradians concentricus Say. Journal of Fishery Sciences of China, 14(5): 778-785.
(in Chinese with English abstract) |
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods, 25(4): 402-408.
DOI:10.1006/meth.2001.1262 |
Mejdoub Z, Fahde A, Loutfi M, et al. 2017. Oxidative stress responses of the mussel Mytilus galloprovincialis exposed to emissary's pollution in coastal areas of Casablanca. Ocean & Coastal Management, 136: 95-103.
DOI:10.1016/j.ocecoaman.2016.11.018 |
Meng X L, Liu P, Li J, et al. 2014. Physiological responses of swimming crab Portunus trituberculatus under cold acclimation: antioxidant defense and heat shock proteins. Aquaculture, 434: 11-17.
DOI:10.1016/j.aquaculture.2014.07.021 |
Naegel L C A, López-Rocha J A. 2007. Effect of temperature on growth of the intertidal purple snail Plicopurpura pansa (Gould 1853) under laboratory conditions. Aquaculture Research, 38(5): 493-497.
DOI:10.1111/j.1365-2109.2007.01691.x |
Ning J H, Chang Y Q, Liu W, et al. 2015. Stress responses to mild and acute temperature decrease for two strains of sea cucumber Apostichopus japonicus. Aquaculture, 448: 552-563.
DOI:10.1016/j.aquaculture.2015.06.035 |
Ning J H, Zou D S, Lu X, et al. 2021. Transcriptomic analyses provide insights into the adaptive responses to heat stress in the ark shells, Scapharca subcrenata. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 38: 100813.
DOI:10.1016/j.cbd.2021.100813 |
Nover L, Scharf K D. 1997. Heat stress proteins and transcription factors. Cellular and Molecular Life Sciences CMLS, 53(1): 80-103.
DOI:10.1007/PL00000583 |
Pan X, Sheng X Q, Zhang W X, et al. 2022. Effects of short-term temperature changes on the physiology, biochemistry and feeding behaviour of the juvenile yellow seahorse-Hippopotamus kuda Bleeker. Aquaculture Research, 53(3): 1084-1097.
DOI:10.1111/are.15649 |
Pechenik J A, Chaparro O R, Lazarus Z M, et al. 2020. Impact of short-term elevated temperature stress on winter-acclimated individuals of the marine gastropod Crepidula fornicata. Marine Environmental Research, 162: 105180.
DOI:10.1016/j.marenvres.2020.105180 |
Qin Y P, Zhang Y H, Li X Y, et al. 2020. Characterization and functional analysis of a caspase 3 gene: evidence that ChCas 3 participates in the regulation of apoptosis in Crassostrea hongkongensis. Fish & Shellfish Immunology, 98: 122-129.
DOI:10.1016/j.fsi.2020.01.007 |
Reddy R K, Mao C H, Baumeister P, et al. 2003. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors-Role of ATP binding site in suppression of caspase-7 activation. Journal of Biological Chemistry, 278(23): 20915-20924.
DOI:10.1074/jbc.M212328200 |
Sanford E, Kelly M W. 2011. Local adaptation in marine invertebrates. Annual Review of Marine Science, 3: 509-535.
DOI:10.1146/annurev-marine-120709-142756 |
Santana P A, álvarez C A, Sáenz-Martínez D E, et al. 2021. New insight to the rol of α-enolase (Eno-1) as immunological marker in rainbow trout fry. Developmental & Comparative Immunology, 123: 104163.
DOI:10.1016/j.dci.2021.104163 |
Schutte B, Nuydens R, Geerts H, et al. 1998. Annexin V binding assay as a tool to measure apoptosis in differentiated neuronal cells. Journal of Neuroscience Methods, 86(1): 63-69.
DOI:10.1016/S0165-0270(98)00147-2 |
Srivastava P. 2002. Roles of heat-shock proteins in innate and adaptive immunity. Nature Reviews Immunology, 2(3): 185-194.
DOI:10.1038/nri749 |
Stillman J H. 2003. Acclimation capacity underlies susceptibility to climate change. Science, 301(5629): 65.
DOI:10.1126/science.1083073 |
Wang Y, Wu Z H, Li H Y, et al. 2013. Analysis and evaluation of nutrition composition in soft tissue of Anadara uropygimelana. Progress in Fishery Sciences, 34(1): 133-139.
(in Chinese with English abstract) |
Wang Z L, Qu Y X, Zhuo X L, et al. 2019. Investigating the physiological responses of Pacific white shrimp Litopenaeus vannamei to acute cold-stress. PeerJ, 7: e7381.
DOI:10.7717/peerj.7381 |
Yampolsky L Y, Schaer T M M, Ebert D. 2014. Adaptive phenotypic plasticity and local adaptation for temperature tolerance in freshwater zooplankton. Proceedings of the Royal Society B: Biological Sciences, 281(1776): 20132744.
DOI:10.1098/rspb.2013.2744 |
Yang C Y, Gao Q, Liu C, et al. 2017. The transcriptional response of the Pacific oyster Crassostrea gigas against acute heat stress. Fish & Shellfish Immunology, 68: 132-143.
DOI:10.1016/j.fsi.2017.07.016 |
Zhang G F, Fang X D, Guo X M, et al. 2012. The oyster genome reveals stress adaptation and complexity of shell formation. Nature, 490(7418): 49-54.
DOI:10.1038/nature11413 |
Zhou Z, Liu Z Q, Wang L G, et al. 2019. Oxidative stress, apoptosis activation and symbiosis disruption in giant clam Tridacna crocea under high temperature. Fish & Shellfish Immunology, 84: 451-457.
DOI:10.1016/j.fsi.2018.10.033 |
Zou D S, Ning J H, Lu X, et al. 2021. Physiological and transcriptional responses to acute and chronic thermal stress in the ark shell Scapharca subcrenata. Frontiers in Marine Science, 8: 739662.
DOI:10.3389/fmars.2021.739662 |
 2023, Vol. 41
2023, Vol. 41



