Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YUAN Ziming, JIANG Wei, SHA Zhongli
- Medaeops Guinot, 1967 (Brachyura, Xanthidae) from China seas, with the resurrection of Medaeops japonicus (
Rathbun, 1898 ) - Journal of Oceanology and Limnology, 41(6): 2403-2417
- http://dx.doi.org/10.1007/s00343-023-2282-7
Article History
- Received Jul. 13, 2022
- accepted in principle Sep. 5, 2022
- accepted for publication Dec. 6, 2022
2 Shandong Province Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
3 Laoshan Laboratory, Qingdao 266237, China;
4 University of Chinese Academy of Sciences, Beijing 100049, China
The genus Medaeops was established by Guinot (1967) in a revision of Medaeus Dana, 1851, and initially contained three species: Medaeops granulosus (Haswell, 1882), M. neglectus (Balss, 1922a), and M. edwardsi Guinot, 1967, of which M. granulosus was selected as the type species. Currently, seven species have been reported in the genus: M. granulosus, M. neglectus, M. edwardsi, M. gemini Davie, 1997, M. merodontos Davie, 1997, M. serenei Ng & McLay, 2007, and M. potens Mendoza, Chong & Ng, 2009. All the seven species were reported from the Indo-West Pacific region; however, only M. granulosus has been reported from China seas. In this study, two morphologically distinct species were found in the populations previously considered as M. granulosus. Materials collected from the South China Sea are here considered as M. granulosus, while individuals of the north coast of China seas are identified as Medaeops japonicus (Rathbun, 1898). M. japonicus was once considered a junior synonym of M. granulosus by Guinot (1967), but herein was proved to be a valid species. In this study, we also found a new record of M. edwardsi from the South China Sea.
DNA barcoding based on mitochondrial cytochrome oxidase I (COI) sequences from Medaeops crabs were used for species delimitation in this genus. The Medaeops species key was also updated.
2 MATERIAL AND METHODSamples were collected from intertidal zones or by trawling from the shallow water area in Chinese waters from Guangxi to Shandong provinces. The specimens were stored in 70% ethanol and deposited at the Museum of Marine Biology, Chinese Academy of Sciences (MBMCAS), Qingdao, China.
Specimens were examined by ZEISS Stemi 2000-c and ZEISS Stemi SV 11 Apo stereomicroscopes and Nikon Eclipse Ci-L microscope. Photographs were taken using a Canon EOS 6D camera with Canon EF 100-mm and Canon MP-E 65-mm lenses or Nikon D800 camera with Nikon AF-S 105-mm lens. Male first gonopods were observed by scanning electron microscopy (SEM; model: Hitachi-S-3400N).
The morphological terminology used in this paper follows that of Dana (1852, 29, text fig), Serène (1984, 12–13, Fig.A, B) and Davie et al. (2015, Fig. 71–2.6). The following abbreviations were used in the text: maximum carapace width (CW); median carapace length (CL); first gonopod of male (G1).
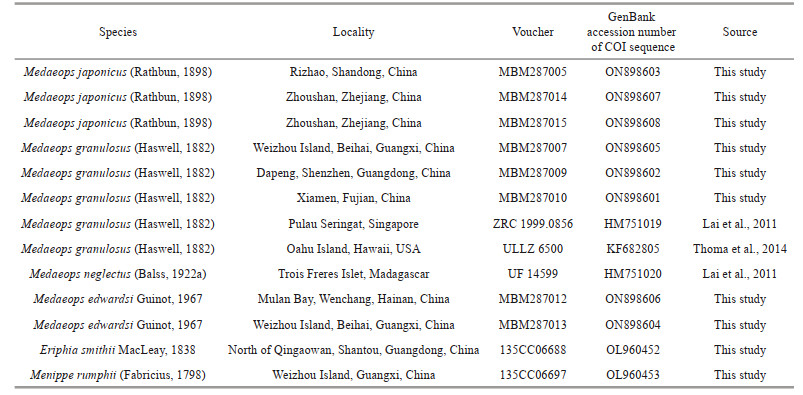
The sequence of COI (524 bp after alignment) was selected for phylogenetic analyses. COI sequences of M. japonicus, M. granulosus, M. edwardsi, and M. neglectus were analyzed, with two Eriphioidea species—Eriphia smithii MacLeay, 1838 and Menippe rumphii (Fabricius, 1798)—selected as outgroups (Table 1). Genomic DNA of Medaeops species was extracted from muscle tissue using the OMEGA EZNA Tissue DNA Kit (USA). Sequences were amplified by polymerase chain reaction (PCR) with the primers dgLCO-1490 and dgHCO-2198 (Meyer, 2003), jgLCO1490 and jgHCO2198 (Geller et al., 2013), and Pano-F and Pano-R (Thoma et al., 2014). A volume of 25 μL was used for the PCR and comprised 1-μL (2–200 ng) genomic DNA template, 1 μL (10 pmol/L) of each primer, 12 μL of 2×PCR Mix (Guangzhou Dongsheng Biotechnology Co., Ltd., China), and 10-μL ultrapure water. The PCR conditions involved an initial denaturation at 94 ℃ for 3 min; 35 cycles of denaturation at 94 ℃ for 30 s, annealing at 48 ℃ for 45 s, and extension at 72 ℃ for 45 s; and a final extension at 72 ℃ for 10 min.
|
The sequences were aligned by MEGA v6.06 using Muscle default settings (Tamura et al., 2013). Phylogenetic trees were reconstructed by Bayesian Inference (BI) and Maximum Likelihood (ML) methods as implemented in MrBayes v3.2.7 (Huelsenbeck and Ronquist, 2001) and W-IQ-TREE (Trifinopoulos et al., 2016), respectively. Genetic divergences of COI between and within species were calculated by a Kimura 2-parameter distance model in MEGA v6.06 (Tamura et al., 2013).
3 TAXONOMYFamily Xanthidae MacLeay, 1838 Subfamily Euxanthinae Alcock, 1898 Genus Medaeops Guinot, 1967
3.1 Medaeops japonicus (Rathbun, 1898) comb. nov.Lophozozymus (Lophoxanthus) bellus var. Leucomanus Miers, 1886: 115, Pl. 11, Fig. 1. [type Lophopanopeus japonicus Rathbun, 1898: 272; Balss, 1922 a: 125. [type locality: Japan].
Lophoxanthus erosus Parisi, 1916: 181, Fig. 4; Menzies, 1948: 21, Pl.4, Fig. 33. [type locality: Tokyo Bay].
Medaeus granulosus Sakai, 1939: 459, Pl. 59, Fig. 1, Pl.90, Fig. 5; 1965: 135, Pl.69, Fig. 2.
Medaeops granulosus Guinot, 1967: 366 (part); Kim, 1973: 382, Fig. 145, Pl. 82, Fig. 110; Miyake, 1983: 109, Pl.37, Fig. 3; Lee, 2012: 123, Figs. 92–94. (Not Haswell, 1882).
Material examined
Yellow Sea: MBM040675, 1♂, CW 16.4 mm, CL 11.2 mm, depth 29 m, 21 October 1958, Agassiz trawl; MBM040674, 1♀, CW 16.1 mm, CL 11.1 mm, depth 35 m, 24 April 1959, Agassiz trawl; MBM287005, 1 ♂, CW 14.2 mm, CL 9.6 mm, 1 ♀, CW 11.6 mm, CL 7.9 mm, Rizhao, Shandong, 22 March 2019, coll. Juhao WANG; MBM287006, 1 ♂, CW 16.1 mm, CL 11.2 mm, 1 ♀, CW 15.9 mm, CL 10.4 mm, Qingdao, Shandong, the first bathing beach, 15 December 2020, coll. Juhao WANG.
Jiaozhou Bay: MBM162986, 1 ♀, CW 8.7 mm CL 6.1 mm, depth 18 m, 4 August 1964, Agassiz trawl; MBM162985, 1♀, CW 12.0 mm, CL 8.3 mm, 35 m, 4 August 1964, Agassiz trawl; MBM287004, 1♂, CW 8.3 mm, CL 5.9 mm, 12 November 2021, grab, coll. Dong DONG.
Zhoushan: MBM287014, 1♂, CW 12.5 mm, CL 9.0 mm, October 2020, coll. Jian CHEN; MBM287015, 1 ♀, CW 15.1 mm, CL 10.3 mm, October 2020, coll. Jian CHEN.
Description
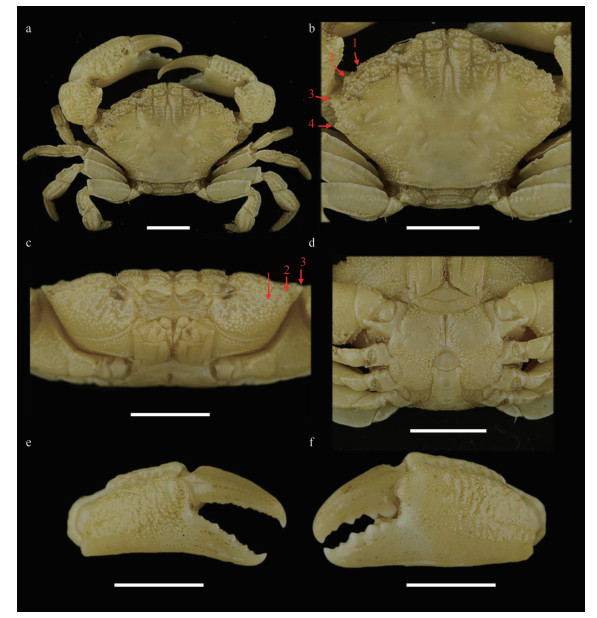
Carapace (Figs. 1a, b, & 2a) hexagonal, approximately 1.5 times wider than long, regions well-defined anteriorly, covered with granules arranged in unclear strings, denser at anterolateral regions 1–5, medial regions 2–4; anterolateral region 6 and posterior regions flat. Frontal region approximately 1/4 of carapace width, divided into two lobes, margin granulated, slightly sinuous, separated from the dorsal margin of orbit by a deep notch. Anterolateral margin armed with four teeth except the outer orbital angle; the first tooth very small and weak, nearly invisible and covered by granules; second tooth wide and low; third tooth most prominent; fourth tooth small but evident. Posterolateral border subequal with anterolateral border; subhepatic covered with dense granules, pterygostomial region separated from subhepatic by a row of granules. Antennule (Fig. 1c) situated transversely; antenna basal segment subrectangle, anterolateral angle produced, orbital hiatus filled by antennal flagellum. Third maxilliped (Fig. 1c) completely covering buccal frame; ischium subquadrate, outer surface covered by granules, with submedian groove; merus subpentagonal, all the outer surface covered with granules. Thoracic sternites (Fig. 1d) granular; the suture between thoracic sternites 1–2 and 2–3 distinct, backward convex, suture between thoracic sternites 3–4 visible, the median line of thoracic sternite 4 distinct and long.
|
| Fig.1 Medaeops japonicus (Rathbun, 1898), male, 16.4 mm×11.2 mm (MBM040675) a. entire specimen; b. carapace; c. frontal view; d. thoracic sternites and pleon; e. right cheliped; f. left cheliped. Arrows indicate anterolateral margin teeth; scale bars=5 mm. |

|
| Fig.2 Carapace and G1 of species of the genus Medaeops a, d, g. Medaeops japonicus (Rathbun, 1898), male, 16.4 mm×11.2 mm (MBM040675); b, e, h. Medaeops granulosus (Haswell, 1882), male, 22.4 mm× 15.4 mm (MBM287008); c, f, i. Medaeops edwardsi Guinot, 1967, male, 32.3 mm×20.5 mm (MBM287013); a–c. carapace; d–f. G1, ventral view, distal part detailed; g–i. G1, entire. Scale bars: a, b: 5 mm; c: 10 mm; d–f: 0.5 mm; g–i: 1 mm. G1: first gonopod of male. |
Chelipeds (Fig. 1e & f) slightly unequal; merus with granules on ventral and outer surface; carpus armed with a blunt tooth on inner angle, dorsal surface with granules forming a reticular relief; palm covered with granules, dorsal surface with irregular areoles, outer surface with a shallow groove near dorsal; dactylus armed with a central ridge and two longitudinal dorsal grooves; finger cutting edges with triangular teeth of different sizes, modified strong molar basal tooth on the large cheliped dactylus, finger tips sharp.
Ambulatory legs (Fig. 1b) flat and merus, carpus, and propodus sculpted by sulcus in lateral and mesial faces; merus anterior edge cristate; carpus with three carina on anterior edge, more or less wavy; propodus slightly cristate; dactylus and subterminal of propodus covered with short setae, dactylus tip sharp, claw-shaped.
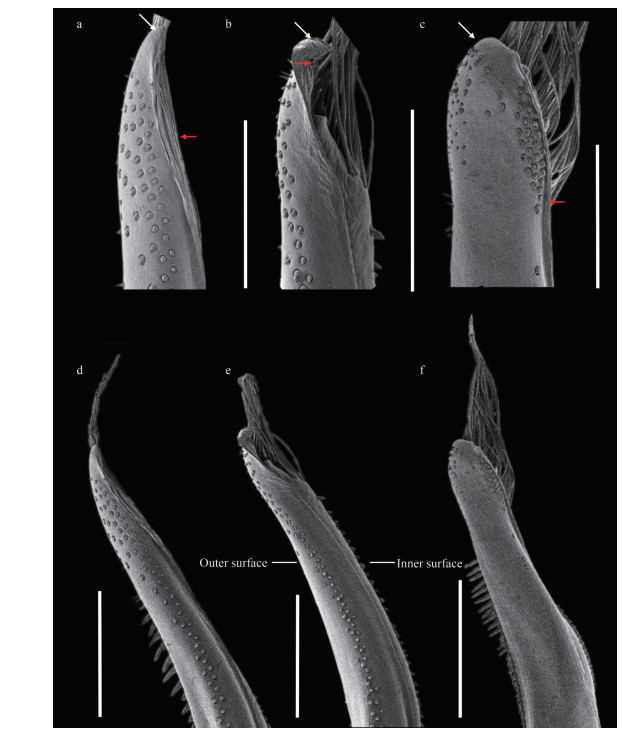
Pleonal somites (Fig. 1d) 3–5 completely fused in male; distant angles of pleonite 6 prominent; telson short. First gonopod (Figs. 2d, 2g, 3a, & 3d) stout, and inner and outer surface with small spines, small spines on outer surface transform into long spines on subterminal; ventral surface with small spines; distal lobe sharp and narrowed, rolled outward, outward-rolled region long; subdistal with long pinnate setae.
|
| Fig.3 SEM of G1 of Medaeops species a, d. Medaeops japonicus (Rathbun, 1898), male, 16.7 mm×11.2 mm (MBM040675); b, e. Medaeops granulosus (Haswell, 1882), male, 22.4 mm×15.4 mm (MBM287008); c, f. Medaeops edwardsi Guinot, 1967, male, 32.3 mm×20.5 mm (MBM287013); a–c. G1 distal part, ventral view; d–f. G1, ventral view. White arrows indicate distal lobe; red arrows indicate outward-rolled region. Scale bars: a, b: 400 μm; c: 500 μm; d, e: 500 μm; f: 1 000 μm. G1: first gonopod of male. |
Live coloration
Overall light yellow-brown, with dark lump and spots; carapace sometimes dark brown, with margins and posterior regions paler in color; fingers black-brown, color somewhat expanding to palm; merus of ambulatory legs with brown bands or spots (Fig. 4).

|
| Fig.4 Live coloration and ecotope of Medaeops japonicus (Rathbun, 1898) from Qingdao a, c. male, 16.1 mm×11.2 mm (MBM287006); b. female, 15.9 mm×10.4 mm (MBM287006); d. environmental sediments of intertidal collection. Scale bars: 1 cm. Photos c and d by Juhao WANG. |
Distribution
The north coast of China seas: from Zhejiang to Shandong; west coast of Korean Peninsula, Kii Peninsula, Izu Peninsula, Sagami Bay, Tokyo Bay. Collected from intertidal to subtidal zones.
Remark
Medaeops japonicus was first reported by Miers (1886) under the name Lophozozymus (Lophocxanthus) bellus var. leucomanus based on materials from Japan collected during the HMS Challenger expedition. Miers cast doubts on the identification because the specimens had the dactylus of chelipeds described as dull in color and not shining white as mentioned in the original description of Xanthodes leucomanus by Lockington (1877). Rathbun (1898) later transferred these Japanese materials to the genus Lophopanopeus Rathbun, 1898 and created a new name (Lophopanopeus japonicus Rathbun, 1898) for them. Another species—Lophoxanthus erosus Parisi, 1916— had been described from material collected off Tokyo Bay. However, Balss (1922b) considered that Lophoxanthus erosus was a junior synonym of Lophopanopeus japonicus according to the description and illustration but did not elaborate further on this. Odhner (1925) listed Lophoxanthus erosus and Lophopanopeus japonicus as synonyms of Leptodius granulosus Haswell, 1882 without explanation, and transferred this species to the genus Medaeus. However, Menzie (1948) noticed that the male first pleopods of Lophoxanthus erosus are rather different from those of Medaeus granulosus and suggested that the species can be considered as valid. In a revision of Medaeus by Guinot (1967), Leptodius granulosus was transferred to the genus Medaeops, Lophoxanthus erosus and Lophopanopeus japonicus were treated as junior synonyms of Medaeops granulosus, and the certain distribution of M. granulosus in Australia, Japan, and China was recognized. Currently, this is the generally accepted view.
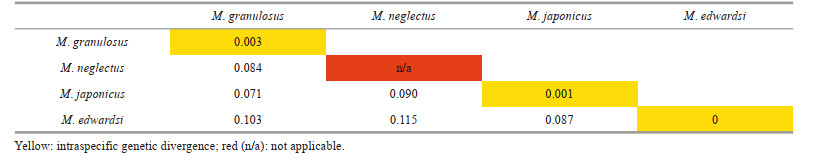
In the Chinese material of "M. granulosus", we found two distinct species with constant morphological and molecular differences. The southern material was identified as M. granulosus (Haswell, 1882), while the northern form showed clearly different characteristics on their obvious reduced first anterolateral tooth, large granules concentrated on anterolateral regions, and the shape of male first gonopod. Although the Miers (1886) material from the Challenger expedition could not be found at the Natural History Museum (Paul F. Clark, personal communication), the material from Zhejiang to Shandong resembles the original description and figure of Lophozozymus (Lophocxanthus) bellus var. Leucomanus and other reports based on materials from the coast of Japan and the Korean Peninsula (Parisi, 1916: Fig. 4; Menzies, 1948: Pl. 4, Fig. 33; Sakai, 1939: Pl. 59, Fig. 1, Pl. 90, Fig. 5; Miyake, 1983: Pl. 37, Fig. 3; Kim, 1973: Fig. 145, Pl. 82, Fig. 110; Lee, 2012: Figs. 92–94). In the molecular analyses based on COI DNA barcoding (Table 2), the southern and northern specimens showed distinct genetic differences (COI sequence divergences 7.1%). No significant genetic difference was found among the northern material (COI sequence similarities 99.9% among ON898603, ON898607, and ON898608). The southern specimens were also genetically almost identical to the sequences of M. granulosus available in the GenBank database (COI sequence similarities 99.7% among the three Chinese specimens ON898601, ON898602, and ON898605, and specimens from Singapore HM751019 and Hawaii KF682805). Based on the above evidence, we resurrect the species Lophopanopeus japonicus Rathbun, 1898 with a new combination name Medaeops japonicus (Rathbun, 1898).
Medaeops japonicus can be distinguished from M. granulosus by: 1) first anterolateral tooth very small, nearly invisible and blended with granules (vs. first anterolateral tooth smaller than others but distinct, still visible even from anterior view in M. granulosus); 2) granules on carapace proportionally larger, more concentrated on anterolateral regions and subhepatic region (vs. granules proportionally smaller, forming more scattered lines in M. granulosus); 3) G1 outer surface with small spines, which transform into long spines on subterminal part (vs. only short and small spines on G1 outer surface in M. granulosus); 4) G1 distal lobe sharp and narrow, outward-rolled region long (vs. G1 distal lobe obtuse, outward-rolled region short in M. granulosus); 5) the black-brown color of fingers slightly expanding to palm (vs. not expanding to palm in M. granulosus).
Medaeops japonicus is also similar to M. edwardsi Guinot, 1967 based on their blurry first anterolateral tooth and G1 with long spines on outer surface and distal lobe with longer outward-rolled region, and similar to M. neglectus (Balss, 1922a) mainly on the shape of G1. M. japonicus can be easily distinguished from M. edwardsi by: 1) second, third, and fourth anterolateral teeth sharp (vs. blunt and more vague in M. edwardsi); 2) carapace surface only with sporadic short setae or smooth (vs. densely covered by short setae on carapace, thoracic sternites, legs, and abdomen in M. edwardsi); 3) merus of ambulatory legs with anterior edge cristate (vs. merus of ambulatory legs with anterior edge not cristate in M. edwardsi); 4) G1 distal lobe sharp and narrowed (vs. distal lobe more obtuse in M. edwardsi). M. japonicus can be distinguished from M. neglectus by: 1) first anterolateral tooth very small, nearly invisible, and blended with granules (vs. first anterolateral tooth small but distinct in M. neglectus); 2) carapace covered with larger granules (vs. carapace with smaller granules in M. neglectus); 3) ambulatory legs merus anterior edge distinct cristate (vs. ambulatory legs merus anterior edge not distinct cristate in M. neglectus) (Barnard, 1950; Forest and Guinot, 1961; Tirmizi and Ghani, 1996; Guinot, 1967; Serène, 1984; Mendoza et al., 2009; Naderloo, 2017).
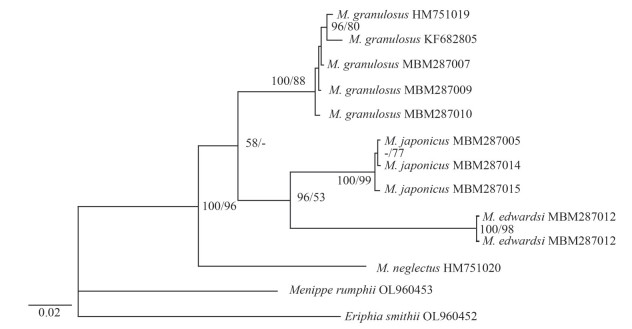
To further delimit the species of genus Medaeops, molecular analyses based on COI DNA barcoding were performed. Interspecific divergences of COI sequences between M. japonicus, M. granulosus, M. edwardsi, and M. neglectus were all greater than 7% (Table 2). Phylogenetic trees (Fig. 5) based on COI sequences were reconstructed by BI and ML methods to reveal the interspecific relatedness. Individuals of the same species clustered together with high support values (100/99 for M. japonicus, 100/88 for M. granulosus, and 100/98 for M. edwardsi). All the species were well supported.
|
|
| Fig.5 Phylogenetic relationships among species of the genus Medaeops based on COI sequences The Bayesian Inference tree is shown, with BI posterior probabilities and Maximum Likelihood bootstrap replications indicated at nodes (PP/BS). |
Leptodius granulosus Haswell, 1882: 61. [type locality: Port Denison, Australia]
Xantho macgillivrayi Miers, 1884: 211, Pl. 20, Fig.C. [type locality: Australia]
Medaeus granulosus Odhner, 1925: 81; Gordon, 1931: 543, Figs. 19, 22A; Stephensen, 1946: 148, Fig. 37A, B; Buitendijk, 1950: 75; Chhapgar, 1957: 430, Pl.9, Figs.g–i. Medaeops granulosus Guinot, 1967: 366, Fig. 40 (part); Serène and Umali, 1972: 65, Figs. 58–59, Pl.7, Figs. 1–2; Serène and Vadon, 1981: 122; Fig. 55B(3), Pl.37(7); Serène, 1984: 91 (key); Dai and Yang, 1991: 295, Fig. 155B(3), Pl.37(7); Davie, 1997: 357 (key); Ng and Davie, 2002: 375 (list), 381; Ng and Mclay, 2007: 48 (key); Mendoza et al., 2009: 51, Fig. 2A–C, Fig. 5A, B, Fig. 6; Mendoza and Ng, 2010: 65; Dev Roy, 2013: 154 (list); Trivedi et al., 2018: 81 (list); Liu, 2008: 795 (list).
Non Medaeus granulosus Balss, 1934: 507; Barnard, 1950: 219, Fig. 41a, Fig. 42a, b; Forest and Guinot, 1961: 56, Fig. 45a, b, Pl.1, Fig. 2 [=Medaeops neglectus Balss, 1922a].
Non Medaeus granulosus Sakai, 1939: 459, Pl.59, Fig. 1, Pl.90, Fig. 5; 1965: 135, Pl.69, Fig. 2 [=Medaeops japonicus (Rathbun, 1898)].
Non Medaeops granulosus Kensley, 1981: 44; Tirmizi and Ghani, 1996: 56–59, Figs. 21, 22; Apel, 2001: 87 [=Medaeops neglectus Balss, 1922a].
Non Medaeops granulosus Guinot, 1967: 366 (part); Kim, 1973: 382, Fig. 145, Pl. 82, Fig. 110; Miyake, 1983: 109, Pl. 37, Fig. 3; Lee, 2012: 123, Figs. 92–94. [=Medaeops japonicus (Rathbun, 1898)].
Material examined
Guangdong: MBM287011, 1 ♂, CW 11.2 mm, CL 7.8 mm, Dapeng, Shenzhen, September 2021; MBM287014, 1 ♂, CW 26.8 mm, CL 17.4 mm, Shenzhen, January 2022; MBM287009, 1 ♂, CW 28.1 mm, CL 18.5 mm, Dapeng, Shenzhen, depth 6 m, February 2021.
Fujian: MBM287008, 1 ♂, CW 22.4 mm, CL 15.4 mm, Xiamen, Jinwan, 23 July 2021, coll. Xu ZHANG; MBM287010, 1 ♂, CW 25.7 mm, CL 17.3 mm, Xiamen, December 2021, coll. Xu ZHANG; MBM205016, 1 ♂, CW 31.1 mm, CL 20.4 mm, 1 ♀, CW 25.3 mm, CL 16.4 mm, 1♀, 20.0 mm×13.7 mm, Xiamen, December 2021, coll. Xu ZHANG.
Guangxi: MBM287007, 1 ♂, CW 19.55 mm, CL 13.32 mm, Weizhou Island, Beihai, December 2019, coll. Juhao WANG.
Description
Carapace (Figs. 6a, 6b, & 2b) hexagonal, approximately 1.5 times wider than long, regions well-defined anteriorly, covered with granules arranged in unclear strings. Frontal region approximately 1/4 the breadth of carapace width, divided into two lobes, margins granulated, slightly sinuous, separated from the dorsal margin of orbit by a deep notch. Anterolateral margin armed with four teeth except the outer orbital angle; the first tooth small but distinct and sharp; second tooth wide, with a sharp tip; third tooth most prominent; fourth tooth small but evident. Posterolateral border subequal with anterolateral border; subhepatic covered with granules, pterygostomial region separated from subhepatic by a row of granules. Antennule (Fig. 6c) situated transversely; antenna basal segment subrectangle, anterolateral angle produced, orbital hiatus filled by antennal flagellum. Third maxilliped (Fig. 6c) completely covering buccal frame; ischium subquadrate, outer surface covered by granules, with submedian groove; merus subpentagonal, all the outer surface covered with granules. Thoracic sternites (Fig. 6d) with granules on anterior part; the suture between thoracic sternites 1–2 and 2–3 distinct, backward convex, suture between thoracic sternites 3–4 visible, the median line of thoracic sternite 4 distinct and long.

|
| Fig.6 Medaeops granulosus (Haswell, 1882), male, 22.4 mm×15.4 mm (MBM287008) a. entire specimen; b. carapace; c. frontal view; d. thoracic sternites and pleon; e. right cheliped; f. left cheliped. Arrows indicate anterolateral margin teeth. Scale bars: 5 mm. |
Chelipeds (Fig. 6e & f) slightly unequal; merus with granules on ventral and outer surface; carpus armed with a blunt tooth on inner angle, dorsal surface with granules and tubercles; palm covered with granules, formed reticular relief on dorsal surface, outer surface with a shallow groove near dorsal; dactylus armed with a central ridge and two longitudinal dorsal grooves; finger cutting edges with blunt teeth in different sizes, modified strong molar basal tooth on the large cheliped dactylus finger tips sharp.
Ambulatory legs (Fig. 6b) stout, covered with granules, merus anterior edge cristate, carpus anterior edge granular, sinuous; propodus and dactylus covered with short setae, dactylus tip sharp, claw-shaped.
Pleonal somites (Fig. 6d) 3–5 completely fused in male; distant angles of pleonite 6 prominent; telson short. First gonopod (Figs. 2e, 2h, 3b, & 3e) stout, and inner, outer, and ventral surface with small spines; distal lobe obtuse, rolled outward, outward-rolled region short; subdistal with long pinnate setae.
Live coloration
Carapace brownish, with margins and posterior regions paler in color; darker brown fingers not expanding to the palm; merus of ambulatory legs with bands or brown spots (Fig. 7).

|
| Fig.7 Live coloration and ecotope of Medaeops granulosus (Haswell, 1882) a–c. male, 31.1 mm×20.4 mm (MBM205016); d, e. male, 22.4 mm×15.4 mm (MBM287008), from Xiamen, Fujian. Scale bar: 1 cm. Photos by Xu ZHANG. |
Distribution
South China Sea: Guangxi, Hainan Island, Guangdong, Hongkong, Fujian, Taiwan Island; Gulf of Oman, west coast of Indian Subcontinent, Phuket Island, Singapore Strait, Philippine Islands, Australia, Hawaii Island. Collected from intertidal zone of rocky seashores.
Remark
Medaeops granulosus (Haswell, 1882) was originally described from Port Denison and Port Molle of eastern Australia. Miers described another species, Xantho macgillivrayi Miers 1884 based on Australian materials collected from Port Molle, Port Curtis, and Facing Island. Odhner (1925) listed X. macgillivrayi as a synonym of Leptodius granulosus Haswell, 1882. Guinot (1967) selected M. granulosus as the type species of Medaeops.
Owing to their very similar morphology, Medaeops granulosus was confused with Medaeops neglectus (Balss, 1922a) for a long time, and Medaeops neglectus is thought to be confined to the Red Sea and the western Indian Ocean (Guinot, 1967; Serène, 1984; Naderloo and Türkay, 2012). According to the literature, the few diagnostic characters that distinguish these species are the anterior edge of the merus of the ambulatory legs has a distinct cristate in M. granulosus (contrast with the not distinct cristate on anterior edge of merus in M. neglectus), and the different shape of G1 (Guinot, 1967; Serène, 1984; Mendoza et al., 2009). The remaining records from the western Indian Ocean still need to be re-examined (Stephensen, 1946; Chhapgar, 1957; Dev Roy, 2013; Trivedi et al., 2018).
Present materials of M. granulosus show significant variations, predominantly on the crests on merus of ambulatory legs (from sharp and strongly protruding to only represented by a slightly extended edge), and the granularity of the carapace (from granules clear and closely packed to barely visible and hidden beneath wrinkles) (Fig. 8). These variations are not accompanied by significant genetic differences (Table 2); consequently, they are regarded as intraspecific.

|
| Fig.8 Medaeops granulosus (Haswell, 1882) a. male, 28.1 mm×18.5 mm (MBM287009); b. male, 19.6 mm×13.3 mm (MBM287007); c. male, 26.8 mm×17.4 mm (MBM287014); d: male, 25.7 mm×17.3 mm (MBM287010). Scale bars: 1 cm. |
Medaeops edwardsi Guinot 1967: 369, Figs. 33, 42 [type locality: Malabar coast]; Serène, 1984: 91 (in key), 92, Fig. 53, Pl.12, Fig.E; Ghani and Tirmizi, 1992: 43, Fig. 4; Tirmizi and Ghani, 1996: 54-56, Fig. 20; Naderloo et al., 2015: 407 (list); Naderloo, 2017: 260, Figs. 21.34; Mendoza, 2021: 467, Fig. 4.
Material examined
MBM287012, 1 ♀, CW 10.1 mm, CL 7.7 mm, Mulan Bay, Wenchang, Hainan, 12 November 2016, coll. Junlong ZHANG et al.; MBM287013, 1♂, CW 32.3 mm, CW 20.5 mm, Weizhou Island, Beihai, Guangxi, December 2019, coll. Juhao WANG.
Diagnosis
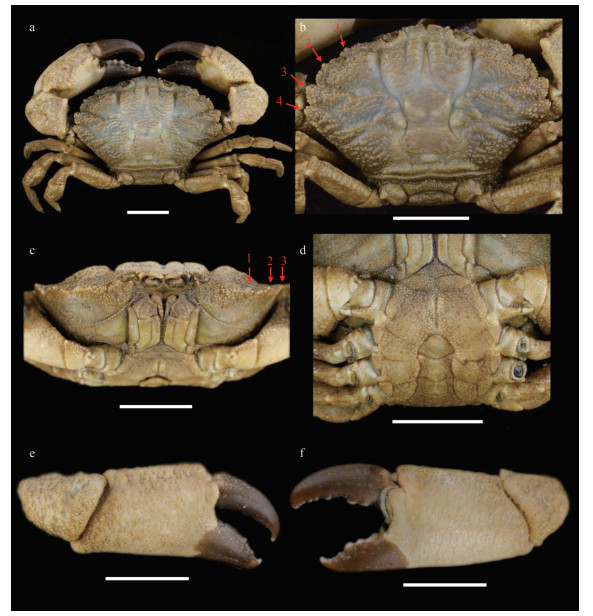
Carapace (Figs. 9a, 9b, & 2c) transversely ovate, approximately 1.5 times wider than long, dorsal flat, with granules arranged in lines, dense short setae in front of the granular lines; anterolateral margin with four blunt and vague teeth; merus of ambulatory legs with anterior edge not cristate (Fig. 9a); G1 (Figs. 2f, 2i, 3c, & 3f) stout, and inner and outer surface with larger spines on subterminal part of outer surface; distal lobe round, rolled outward, outward-rolled region long and narrow, subdistal with long pinnate setae.
|
| Fig.9 Medaeops edwardsi Guinot, 1967, male (32.3 mm×20.5 mm) (MBM287013) a. entire specimen; b. carapace; c. frontal view; d. thoracic sternites and pleon; e. right cheliped; f. left cheliped. Arrows indicate anterolateral margin teeth. Scale bars: 10 mm. |
Distribution
South China Sea: Guangxi, Hainan Island; Madagascar Island, Gulf of Oman, west coast of Indian subcontinent, Singapore Strait, and Australia. Collected from intertidal zone.
Remark
This species was first reported on the Malabar Coast and the paratype is probably from Madagascar (see Guinot, 1967). Another record of M. edwardsi was found among the type material of M. granulosus, which was collected from Queensland (Mendoza et al., 2009).
The original description of this species given by Guinot (1967) is detailed and accurate. This is the first report of Medaeops edwardsi in Chinese waters. The current materials are generally consistent with previous descriptions (Guinot, 1967; Serène, 1984; Ghani and Tirmizi, 1992; Naderloo, 2017), except for the larger male, who shows prominent and well-defined anterolateral teeth separated by deep v-shaped notches (Figs. 9b & 2c). In previous descriptions, the anterolateral margin of M. edwardsi is formed by four denticulate lobes separated by deep cracks but not formed by teeth (Guinot, 1967, Fig. 33; Serène, 1984, Pl. 12, Fig. E; Ghani and Tirmizi, 1992, Fig. 4; Tirmizi and Ghani, 1996, Fig. 20; Naderloo, 2017, Figs. 21, 34; Mendoza, 2021, Fig. 4). In contrast, the anterolateral margin of the smaller female is consistent with previous descriptions. Considering there are no genetic differences between the two individuals (Table 2, COI sequence similarity is 100% between ON898604 and ON898606), such difference is considered as a sexual or ontogenetic difference.
Considering the many name changes in the genus Medaeops, the geographical distribution of the three species in the China seas are now updated (Fig. 10) and a key to the species of the genus Medaeops is provided below.

|
| Fig.10 Worldwide distribution of M. granulosus, M. japonicus, and M. edwardsi |
Map review No. GS(2016)1665.
4 KEY TO THE SPECIES OF MEDAEOPS (ADAPTED FROM NG & MCLAY, 2007)1 Carapace anterolateral margin teeth only weakly developed on an evenly convex margin................................... M. edwardsi Guinot, 1967
-Carapace anterolateral teeth prominent, broad.................................................................................... 2
2 Ambulatory legs distinctly carinate along anterior margins................................................................. 3
-Ambulatory legs not distinctly carinate along anterior margins................................................................. 5
3 Front distinct prominent; anterior half of carapace is much shorter than posterior half; anterolateral teeth developed and prominent............................... M. potens Mendoza, Chong & Ng, 2009
-Front not prominent, anterior half of carapace subequal with posterior half........................................... 4
4 First anterolateral tooth of carapace weak and small; G1 inner surface with long spines on subterminal part, distal lobe sharp and narrow, outward-rolled region long..........................................M. japonicus (Rathbun, 1898)
-First anterolateral tooth of carapace small but distinct; G1 inner surface only with short spines, distal lobe obtuse, outward-rolled region short.............................. M. granulosus (Haswell, 1882)
5 Carapace medial regions 4 distinct; ambulatory legs with row of spaced teeth along anterior margins of merus............................. M. merodontos Davie, 1997
-Carapace medial regions 4 not distinct; ambulatory legs with anterior margins of merus smooth or with at most large granules....................... 6
6 Ambulatory legs relatively long; carapace evenly granular. Frontal lobes separated by a broad notch............................................ M. gemini Davie, 1997
-Ambulatory legs relatively short; carapace with granules arranged in clumps especially on anterior third; frontal lobes separated by a narrow slit or notch; relatively short ambulatory legs...................... 7
7 Carapace regions well marked on anterior 2/3; pleonal segment 6 with concave margins; G1 stout...................................... M. neglectus (Balss, 1922)
-Carapace regions well marked only on anterior 1/3; pleonal segment 6 with straight parallel margins; G1 slender.....................................................M. serenei Ng & McLay, 2007
5 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
6 ACKNOWLEDGMENTWe would like to thank Dong DONG, Junlong ZHANG (Institute of Oceanology, Chinese Academy of Sciences), and Juhao WANG and Xu ZHANG for the specimen collection, and Jian CHEN and Xingle GUO (Zhejiang Ocean University) for kindly providing the Zhoushan specimens. We are also very grateful to Juhao WANG and Xu ZHANG from Institute of Zoology, Chinese Academy of Sciences for providing photos of live crabs. We would like to thank Wei LIU and Yuanyuan SUN (Institute of Oceanology, Chinese Academy of Sciences) for their help in the SEM observation.
Apel M. 2001. Taxonomie und Zoogeographie der Brachyura, Paguridea und Porcellanidae (Crustacea: Decapoda) des Persisch-Arabischen Golfes. Biologie und Informatik, Johann Wolfgang Goethe-Universität, Frankfurt am Main. p. 1-260.
|
Balss H. 1922a. Diagnosen neuer japanischer Decapoden. Zoologischer Anzeiger, 54(1-2): 1-6.
|
Balss H. 1922b. Ostasiatische Decapoden. Ⅳ. Die Brachyrhynchen (Cancridea). Archiv für Naturgeschichte, 88(11): 94-166.
|
Balss H. 1934. Sur Quelques Décapodes brachyoures de Madagascar. Faune des Colonies Françaises, 5(8): 501-528.
|
Barnard K H. 1950. Descriptive catalogue of South African Decapod Crustacea (Crabs and Shrimps). Annals of the South African Museum, 38: 1-837.
|
Buitendijk A M. 1950. Note on a collection of Decapoda Branchyura from the coasts of Mexico, includung the description of a new genus and species. Zoologische Mededelingen, 30(17): 269-282.
|
Chhapgar B F. 1957. On the marine crabs (Decapoda, Brachyura) of Bombay State. Part Ⅰ. Journal of the Bombay Natural History Society, 54(2): 399-439.
|
Dai A Y, Yang S L. 1991. Crabs of the China Seas. China Ocean Press, Beijing and Springer Verlag, Berlin, Heidelberg, New York, Tokyo, 1-21, 1-608, pls. 1-74.
|
Dana J D. 1852. Crustacea. Part Ⅰ. United States Exploring Expedition. During the years 1838, 1839, 1840, 1841, 1842. Under the command of Charles Wilkes, U. S. N. Vol. 13. Sherman C, Philadelphia. 685p, https://doi.org/10.5962/bhl.title.69333.
|
Davie P J F. 1997. Crustacea Decapoda: deep water Xanthoidea from the south-western Pacific and the western Indian Ocean. In: Crosnier A ed. Résultats des Campagnes MUSORSTOM, Volume 18. Mémoires du Muséum National d'Histoire Naturelle 176, Paris. p. 337-387.
|
Davie P J F, Guinot D, Ng P K L. 2015. Anatomy and functional morphology of Brachyura. In: Castro P, Davie P J F, Guinot D et al. eds. Treatise on Zoology-Anatomy, Taxonomy, Biology. The Crustacea, Volume 9 Part C (2 Vols). Brill, Leiden. p. 11-163, https://doi.org/10.1163/9789004190832_004.
|
Dev Roy M K. 2013. Diversity and distribution of marine brachyuran crab communities inhabiting west coast of India. In: Venkataraman K, Sivaperuman C, Raghunathan C eds. Ecology and Conservation of Tropical Marine Faunal Communities. Springer, Berlin, Heidelberg. p. 147-169, https://doi.org/10.1007/978-3-642-38200-0_10.
|
Fabricius J C. 1798. Supplementatum Entomologiae Systematicae. Proft & Storch, Hafniae. p. 1-572.
|
Forest J, Guinot D. 1961. Crustacés Décapodes Brachyoures de Tahiti et des Tuamotu. In: Expédition Français sur les Récifs Coralliens de la Nouvelle-Calédonie. A. Lahure, Paris. p. 1-195.
|
Geller J, Meyer C, Parker M, et al. 2013. Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit Ⅰ for marine invertebrates and application in all-taxa biotic surveys. Molecular Ecology Resources, 13(5): 851-861.
DOI:10.1111/1755-0998.12138 |
Ghani N, Tirmizi N M. 1992. Occurrence of four xanthid (Brachyura) crabs in Karachi waters (Northern Arabian Sea). Pakistan Journal of Marine Sciences, 11: 37-47.
|
Gordon I. 1931. Brachyura from the coasts of China. Zoological Journal of the Linnean Society, 37(254): 525-558.
DOI:10.1111/j.1096-3642.1931.tb02365.x |
Guinot D. 1967. Recherches préliminaires sur les groupements naturels chez les Crustacés Décapodes Brachyoures. Ⅱ. Les anciens genres Micropanope Stimpson et Medaeus Dana. Bulletin du Muséum National d'Histoire naturelle, 39(2): 345-374.
|
Haswell W A. 1882. Catalogue of the Australian Stalk- and Sessile-Eyed Crustacea. Australian Museum, Sydney. p. 1-324, https://doi.org/10.5962/bhl.title.1948.
|
Huelsenbeck J P, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17(8): 754-755.
DOI:10.1093/bioinformatics/17.8.754 |
Kensley B F. 1981. On the zoogeography of southern African decapod Crustacea, with a distributional checklist of the species. Smithsonian Contributions to Zoology, 338: 1-64.
DOI:10.5479/si.00810282.338 |
Kim H S. 1973. A catalogue of Anomura and Brachyura from Korea. In: Illustrated Encyclopedia of Fauna and Flora of Korea. Vol. 14. Samhwa Publishing Company, Seoul. p. 1-694.
|
Lai J C Y, Mendoza J C E, Guinot D, et al. 2011. Xanthidae Macleay, 1838 (Decapoda: Brachyura: Xanthoidea) systematics: a multi-gene approach with support from adult and zoeal morphology. Zoologischer Anzeiger-A Journal of Comparative Zoology, 250(4): 407-448.
DOI:10.1016/j.jcz.2011.07.002 |
Lee S K. 2012. Systematic study on the Korean pilumnoid and xanthoids (Crustacea: Decapoda: Brachyura) based on morphology and molecular data. Laboratory of Systematics and Molecular Evolution, School of Biological Sciences, The Graduate School, Seoul National University, Seoul. 343p.
|
Liu R Y. 2008. Checklist of Marine Biota of China Seas. Science Press, Beijing, China. 1267p. (in Chinese)
|
Lockington W N. 1877. Remarks on the Crustacea of the Pacific coast, with descriptions of some new species. Proceedings of the California Academy of Sciences, 7: 28-36.
|
MacLeay W S. 1838. On the Brachyurous Decapod Crustacea brought from the Cape by Dr. Smith. In: Smith A ed. Illustrations of the Annulosa of South Africa; being a portion of the objects of natural history chiefly collected during an expedition into the interior of South Africa, under the direction of Dr. Andrew Smith, in the years 1834, 1835, and 1836; fitted out by "The Cape of Good Hope Association for Exploring Central Africa". Smith, Elder, and Co., London. p. 53-71.
|
Mendoza J C E. 2021. Marine crabs new to Singapore, with a description of a new species of intertidal xanthid crab of the genus Macromedaeus Ward, 1942 (Crustacea: Decapoda: Brachyura). Raffles Bulletin of Zoology, 69: 463-480.
|
Mendoza J C E, Chong V C, Ng P K L. 2009. A new xanthid crab of the genus Medaeops Guinot, 1967, from Peninsular Malaysia, with a note on Leptodius granulosus Haswell, 1882 (Crustacea: Decapoda: Brachyura: Xanthidae). Zootaxa, 2297(1): 44-54.
DOI:10.11646/zootaxa.2297.1.4 |
Mendoza J C E, Ng P K L. 2010. The euxanthine crabs (Crustacea: Brachyura: Xanthidae) of the Philippines. The Raffles Bulletin of Zoology, 58(1): 57-74.
|
Menzies R J. 1948. A Revision of the Brachyuran Genus Lophopanpoeus. Allan Hancock Foundation Publications, Occasional Papers, 4: 1-45.
|
Meyer C P. 2003. Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biological Journal of the Linnean Society, 79(3): 401-459.
DOI:10.1046/j.1095-8312.2003.00197.x |
Miers E J. 1884. Crustacea (Brachyura). In: Report on the Zoological Collections made in the Indo-Pacific Ocean during the Voyage of H. M. S. Alert 1881-1882. PartI. The Collections from Melanesia. Part Ⅱ. The Collections from the Western Indian Ocean. British Museum (Natural History), London. 8(1): 178-322, pls. 18-32.
|
Miers E J. 1886. Report on the Brachyura collected by H.M. S. Challenger during the years 1873-1876. In: Murray J ed. Zoology. Report on the Scientific Results of the Voyage of H. M. S. Challenger During the Years 1873-76 Under the Command of Captain George S. Nares, R. N., F. R. S. and the Late Captain Frank Tourle Thomson, R. N. Wyville Thomson, C. and J. Murray (series eds.). Neill and Company, Edinburgh. 17: 1-362, pls. 29.
|
Miyake S. 1983. Japanese Crustacean Decapods and Stomatopods in color. Vol. Ⅱ: Brachyura (Crabs). Hoikusha, Osaka. 277p.
|
Naderloo R. 2017. Atlas of Crabs of the Persian Gulf. Springer, Verlag, Heidelberg. 440p, https://doi.org/10.1007/978-3-319-49374-9.
|
Naderloo R, Tuerkay M. 2012. Decapod crustaceans of the littoral and shallow sublittoral Iranian coast of the Persian Gulf: faunistics, biodiversity and zoogeography. Zootaxa, 3374(1): 1-67.
DOI:10.11646/zootaxa.3374.1.1 |
Naderloo R, Ebrahimnezhad S, Sari A. 2015. Annotated checklist of the decapod crustaceans of the Gulf of Oman, northwestern Indian Ocean. Zootaxa, 4028(3): 397-412.
DOI:10.11646/zootaxa.4028.3.5 |
Ng P K L, Davie P J F. 2002. A checklist of the brachyuran crabs of Phuket and western Thailand. Phuket Marine Biological Center Special Publication, 23(2): 369-384.
|
Ng P K L, Mclay C L. 2007. Two new species of deep-water xanthid crabs of the genera Euryxanthops garth & Kim, 1983, and Medaeops guinot, 1967 (Crustacea: Decapoda: Brachyura: Xanthidae) from New Zealand. Zootaxa, 1505(1): 37-50.
DOI:10.11646/zootaxa.1505.1.3 |
Odhner T. 1925. Monographierte Gattungen der Krabbenfamilie Xanthidae. l. Göteborgs Kungliga Vetenskaps-och Vitterhets-Samhälles Handlingar, 29(1): 3-92.
|
Parisi B. 1916. I Decapodi giapponesi del Museo di Milano Ⅳ. Cyclometopa. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano, 55: 153-170.
|
Rathbun M J. 1898. The Brachyura of the biological expedition to the Florida Keys and the Bahamas in 1893. Bulletin from the Laboratories of Natural History, State University of Iowa, 4(3): 250-294.
|
Sakai K. 1965. The Crabs of Sagami Bay: Collected by His Majesty the Emperor of Japan. Maruzen Co. Ltd., Tokyo. 206p.
|
Sakai T. 1939. Studies on the Crabs of Japan Ⅳ. Brachygnatha, Brachyrhyncha. Vol. 3. Yokendo Co., Ltd., Tokyo. p. 365-741.
|
Serène R. 1984. Xanthoidea: Xanthidae et Trapeziidae. Addendum: Carpiliidae et Menippidae. Faune Tropicale, 24: 1-349.
|
Serène R, Umali A F. 1972. The family Raninidae and other new and rare species of brachyuran decapods from the Philippines and adjacent regions. Philippine Journal of Science, 99(1-2): 21-105.
|
Serène R, Vadon C. 1981. Crustacés Décapodes: Brachyoures Liste préliminaire, description de formes nouvelles et remarques taxonomiques. In: Résultats des Campagnes MUSORSTOM. I-Philippines (18-28 Mars 1976). Mémoires ORSTOM. Vol. 91. Éditions de l' Office de la Recherche Scientifique et Technique d' Outre-Mer, Paris. p. 117-140.
|
Stephensen K. 1946. The Brachyura of the Iranian Gulf with an appendix: The male pleopoda of the Brachyura. Danish Scientific Investigations in Iran, 4: 57-237.
|
Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6:molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12): 2725-2729.
DOI:10.1093/molbev/mst197 |
Thoma B P, Guinot D, Felder D L. 2014. Evolutionary relationships among American mud crabs (Crustacea: Decapoda: Brachyura: Xanthoidea) inferred from nuclear and mitochondrial markers, with comments on adult morphology. Zoological Journal of the Linnean Society, 170(1): 86-109.
DOI:10.1111/zoj.12093 |
Tirmizi N M, Ghani N. 1996. Crustacea: Brachyura, Brachyrhyncha Part 1 (Xanthidae, Goneplacidae, Pinnotheridae, Ocypodidae, Grapsidae), Centre of Excellence in Marine Biology, University of Karachi. Marine Fauna of Pakistan, 5: 1-188.
|
Trifinopoulos J, Nguyen L T, Von haeseler A, et al. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research, 44(W1): W232-W235.
DOI:10.1093/nar/gkw256 |
Trivedi J N, Trivedi D J, Vachhrajani K D, et al. 2018. An annotated checklist of the marine brachyuran crabs (Crustacea: Decapoda: Brachyura) of India. Zootaxa, 4502(1): 1-83.
DOI:10.11646/zootaxa.4502.1.1 |
 2023, Vol. 41
2023, Vol. 41


