Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LUO Shengyu, LIU Cheng, GAO Xinming, WANG Jingqian, ZHANG Yibo, DING Jie, HOU Congcong, ZHU Junquan, LOU Bao, SHEN Weiliang, WU Xiongfei, ZHANG Chundan
- Environmental hypoxia induces apoptosis in large yellow croaker Larimichthys crocea via both intrinsic and extrinsic pathways
- Journal of Oceanology and Limnology, 41(6): 2429-2443
- http://dx.doi.org/10.1007/s00343-022-2260-5
Article History
- Received Jul. 4, 2022
- accepted in principle Aug. 19, 2022
- accepted for publication Oct. 19, 2022
2 State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, Zhejiang Academy of Agricultural Sciences, Hangzhou 310021, China;
3 State Key Laboratory of Large Yellow Croaker Breeding, Ningbo Academy of Oceanology and Fishery, Ningbo 315012, China
Dissolved oxygen (DO), i.e., molecular oxygen dissolved in aquatic environments, is the primary oxygen source for various aquatic organisms. The diffusion rate of oxygen in water is only 1/10 000 of air, and the oxygen content in the same volume of water at the same specific pressure is only 1/30 of air (Vuori et al., 2004; Sun et al., 2011). Unlike the terrestrial environment, hypoxia with DO < 2 mg/L occurs frequently in aquatic environments (Diaz, 2001). It is worrying that the frequency and severity of hypoxia in the marine environment is expected to continue to increase due to climate change, environmental pollution, and elevated density of aquatic organisms (Breitburg et al., 2018). Hypoxia can distort the homeostasis of the internal environment of fish, leading to reduced feed intake, growth rate and reproduction, and even death (Martínez et al., 2011; Schulte, 2014).
Under normal physiological conditions, apoptosis can remove damaged cells from the organism to maintain the homeostasis of the internal environment. In addition, when an organism is under excessive stress or pathological conditions, pro-apoptotic factors are overactivated, triggering excessive apoptosis or even secondary necrosis, eventually leading to damage and the death of the organism (Ren et al., 2006; Wang et al., 2006). Apoptosis may be triggered by numerous stressors, such as hypoxia, nutrient deprivation, high or low temperature, high or low pH, changes in osmotic pressure, and protein misfolding (Grilo and Mantalaris, 2019). In mammals, intrinsic (also known as the mitochondrial pathway) and extrinsic (also known as the death receptor pathway) pathways are the two important pathways of apoptosis induced by hypoxia stress (Pan et al., 2014; Lohberger et al., 2016). For instance, under hypoxia stress in cells, electrons in the mitochondrial electron transport chain (ETC) have been shown to escape before their transfer to complex Ⅳ and react with O2 to generate large amounts of reactive oxygen species (ROS) (Halliwell, 1992; Semenza, 2011), which lead to DNA damage (Hammond et al., 2002), altered mitochondrial membrane permeability (Crompton, 1999; Hausenloy et al., 2003), and the release of apoptotic proteins such as cytochrome C (CytC). It is worth noting that aquatic environments are more susceptible to hypoxia than terrestrial environments. The relevance of apoptosis to hypoxia response in aquatic animals has received much attention, and studies on the effects of hypoxia stress on apoptosis in fish have been reported in Hypophthalmichthys molitrix (Zhao et al., 2016; Ding et al., 2018), Danio rerio (Li et al., 2017), Megalobrama amblycephala (Wu et al., 2016), Mudskipper boleophthalmus (Ren et al., 2018), Micropterus salmoides (Zhao et al., 2020), Micropogonias undulatus (Ondricek and Thomas, 2018), Oryzias melastigma (Tse et al., 2015), and Acipenser shrenckii (Lu et al., 2005).
Apoptosis is mainly mediated by the mitochondrial pathway and death receptor pathway. The mitochondrial pathway has been reported to be predominantly regulated by the B-cell lymphoma/leukemia-2 (Bcl-2) protein family (Chao and Korsmeyer, 1998). In normal cells, members of the Bcl-2 anti-apoptotic protein have been found to antagonize the pro-apoptotic effects of Bcl-2 associated X (Bax) and Bcl-2 antagonist/killer (Bak). In intrinsic apoptotic cells, the transcription and translation of members of the BH3-only subfamily have been shown to both enhance and inhibit the anti-apoptotic function of Bcl-2 and indirectly activate Bak and Bax (Youle and Strasser, 2008), which induce a decrease in mitochondrial membrane potential (MMP) and alterations in outer mitochondrial membrane permeability (Knudson et al., 1995; Wei et al., 2001; Reed, 2006). These changes are known to lead to the translocation of CytC from the mitochondria to the cytoplasm (Liu et al., 1996; Ow et al., 2008), where it binds to apoptotic protease activating factor-1 (Apaf1) and recruits procaspase-9 to form the apoptosome (Yuan and Akey, 2013). Subsequently, complex-bound procaspase-9 is cleaved into active caspase-9 (Casp9), activating in turn downstream caspase-3 (Casp3) and caspase-7 (Casp7), which can act as apoptotic "executors", cleaving thousands of cellular substrates (Acehan et al., 2002). In addition, following the altered mitochondrial membrane permeability, the apoptosis-inducing factor (AIF) translocates from the mitochondria to the cytoplasm and enters the nucleus to execute caspase-independent apoptotic programs (Susin et al., 1999). The death receptor pathway is known to be activated by death ligands such as Fas ligand (FasL), tumor necrosis factor-α (Tnf-α), and tumor necrosis factor (TNF) superfamily member 10 (Tnfsf10, also known as TRAIL), which bind to specific receptors on the cell surface to generate the death-inducing signaling complex (DISC), which cleaves procaspase-8 into active caspase-8 (Casp8) (Ashkenazi and Dixit, 1998). Consequently, activated Casp8 cleave procaspase-3 to its active Casp3 form that performs the apoptotic program (Tummers and Green, 2017). Thus, the intrinsic and extrinsic pathways of apoptosis are interlinked and work together to induce apoptosis.
The large yellow croaker Larimichthys crocea is an economically important fish distributed along the southeast coast of China. However, few studies have investigated the effects of stress conditions on apoptosis in L. crocea. Only Wang et al. (2020b) reported the effects of treatment with hydrogen peroxide (H2O2) on oxidative stress and apoptosis in large yellow croaker head kidney cells. Studies on the effects of hypoxia stress on apoptosis in L. crocea have not been reported. Here, we examined for the first time the effect of hypoxia stress on apoptosis in L. crocea both in vivo and in vitro to accumulate basic biological information that would facilitate the elucidation of the mechanism of hypoxia response in fish.
2 MATERIAL AND METHOD 2.1 Fish experiments and sample collectionLarge yellow croakers (length 15.90±1.52 cm, body weight 63.61±6.63 g) were commercially obtained from Fufa Aquatic Products Co., Ltd. (Ningde, China). The fish were domesticated in aerated natural seawater (DO 7.8±0.5 mg/L; salinity 29; temperature 22±0.5 ℃; pH 8.1) for two weeks prior to the experiment. A total of 240 fish were randomly divided into six tanks (800 L each). Three of them were used as the hypoxia groups, whereas the remaining three tanks were used as the normoxia group. For the hypoxia group, the DO in each tank was lowered to 2.0 mg/L by infusing nitrogen within 10 min of beginning the experiment and then maintained at 2.0±0.1 mg/L for 96 h. For the normoxia group, treatment conditions were in agreement with the domestication conditions. The HACH DO probe system (HACH LDO II, HACH, Loveland, CO, USA) was used to monitor and maintain DO in real-time. The samples were collected at 0, 3, 6, 12, 24, 48, and 96 h after starting the experiment. At each time point, three fish were collected from each tank and sacrificed. Dissected liver samples were stored at -80 ℃ or fixed with 4% paraformaldehyde (PFA) solution.
2.2 Cell culture and hypoxia challengeThe large yellow croaker fry (LYCF) cell line was kindly provided by Dr. Youhua HUANG from the South China Agricultural University (Guangzhou, China). LYCF cells were cultured in Leibovitz's-15 Medium (Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco) and 200 µg/mL of penicillin-streptomycin (Gibco). For the normoxia groups, cells were cultured at 27 ℃ in an incubator. For the hypoxia groups, LYCF cells were cultured under hypoxia conditions at 1% O2 and 99% N2 in a MIC-101 modular incubator (Billups Rothenberg Inc., Del Mar, CA, USA) for 0, 3, 6, 12, 24, and 48 h.
2.3 Detection of apoptotic ratesThe annexin V-FITC/PI apoptosis detection kit (Beyotime, Shanghai, China) was used to stain LYCF cells according to the manufacturer's instructions. Stained cells were immediately examined and photographed using a confocal laser microscope (LSM880, Carl Zeiss Meditec, Jena, Germany) or subjected to flow cytometry (Becton Dickinson, San Jose, CA, USA) to detect the apoptotic rates. Acquired data were analyzed using the FlowJo 10 software (Treestar, Ashland, USA).
2.4 TdT mediated dUTP nick end labeling (TUNEL) immunofluorescence stainingThe one-step TUNEL apoptosis assay kit (Beyotime) was used to perform TUNEL immunofluorescence staining on frozen sectioned liver samples and cells seeded in confocal dishes according to the manufacturer's instructions. Immunostaining images were obtained using a laser confocal microscope (LSM880, Carl Zeiss, Jena, Germany).
2.5 Transmission electron microscopy (TEM)Preparation and staining of ultrathin sections of LYCF cells after 24, 48, and 72 h of hypoxia were performed according to the method described by Quignard et al. (2012). The sections were examined and photographed using a transmission electron microscope (TEM; H-7650; Hitachi Medical Co., Chiba, Japan).
2.6 Detection of mitochondrial membrane potentialThe MMP of LYCF cells was assessed using the potentiometric dye tetramethyl rhodamine methyl ester (TMRM; MedChem Express, Shanghai, China) at a final concentration of 0.5 μmol/L for 20 min at 27 ℃.
2.7 Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)Expression patterns of Bax, Bcl-2, and Casp3 were assessed with RT-qPCR using a LightCycler 480 instrument (Roche Diagnostics, Penzberg, Germany). The primers used for RT-qPCR are listed in Table 1. cDNA synthesis and RT-qPCR analysis were performed as previously described (Luo et al., 2019).
The cleavage activity of Casp3/7 in LYCF cells was determined using a GreenNucTM Caspase-3/7 assay kit for live cells (Beyotime) according to the manufacturer's instructions.
2.9 Measurement of activities of caspase-3, 8, and 9The activities of Casp3, Casp8, and Casp9 were measured both in vivo and in vitro using Casp3, Casp8, and Casp9 activity assay kits (Beyotime), respectively, according to the manufacturers' instructions.
2.10 Western blotWhole tissue or cell lysates were prepared using RIPA buffer (Beyotime) containing the protease inhibitors phenylmethanesulfonyl fluoride (PMSF; Beyotime). Protein concentration was measured using a bicinchoninic acid protein assay kit (Cwbio, Beijing, China). After boiling, the supernatants were subjected to sodium dodecyl sulfate polyAcrylamide gel electrophoresis (20-μg protein per lane) and transferred to polyvinylidene fluoride membranes. After blocking with 5% non-fat milk, membranes were successively incubated with primary and horseradish peroxidase-conjugated secondary antibodies before visualizing bands using a chemiluminescence image analysis system (Tanon 5200, Tanon, Shanghai, China).
2.11 Nucleoplasm distribution of apoptosis-inducing factorThe nuclear and cytoplasmic protein extraction kit (Beyotime) was used to isolate nuclear and cytoplasmic proteins according to the manufacturer's instructions. Extracted nuclear and cytoplasmic proteins were subjected to western blot analysis. For the subcellular localization of AIF, LYCF cells seeded in confocal dishes were washed with phosphate buffer saline (PBS) twice and fixed with 4% PFA for 30 min at 25 ℃. After washing with PBS and permeabilization with phosphate-buffered saline with 0.3% Triton X-100-containing buffered saline (PBST), sections were blocked with 5% FBS in PBS and incubated with rabbit anti-human AIF polyclonal antibody (1꞉ 100; Beyotime) overnight at 4 ℃. The next day, sections were incubated with Alexa Fluor 555 labeled donkey anti-rabbit IgG (1꞉500; Beyotime) at 37 ℃ for 1 h. Nuclei were counterstained with 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI, Beyotime). Stained sections were imaged using a confocal laser microscope (LSM880, Carl Zeiss, Jena, Germany).
2.12 Statistical analysisData are expressed as mean±standard error of the means. Statistical analyses were carried out with SPSS software, version 21.0 (IBM, Armonk, NY, USA). Significant differences between 2 groups were determined using the two-tailed independent samples t-test. Significant differences among 3 or more groups were determined using one-way analysis of variance (ANOVA), followed by Tukey's post hoc test.
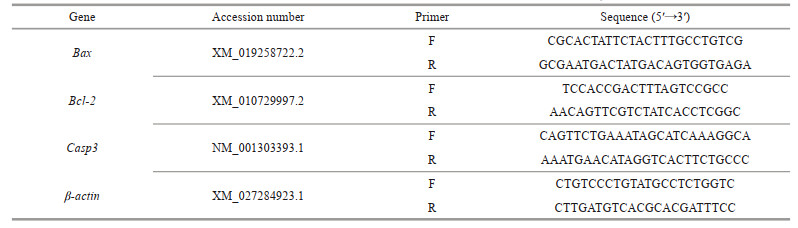
3 RESULT 3.1 Change in the apoptotic rate of LYCF cells under hypoxia stressAfter 24 h of hypoxia stress, LYCF cells were double-stained with annexin V-FITC/PI. We found that the positive signal of annexin V and PI in the hypoxia group was significantly enhanced compared with that in the normoxia group (Fig. 1a). In addition, flow cytometry analysis revealed that the apoptotic rate of LYCF cells in the normoxia group was 8.99%, whereas that of LYCF cells in the hypoxia group was significantly increased to 35.47% (Fig. 1b–c).
|
| Fig.1 Change in the apoptotic rate of LYCF cells after 24 h of hypoxia stress a. annexin V-FITC/PI staining results of LYCF cells after 24 h of hypoxia stress; b & c. the results of annexin V-FITC/PI flow cytometry of LYCF cells after 24 h of hypoxia stress. The scattered dots in the first quadrant (Q1) of the "cross" gate in (b) represent naked cells, those in the second quadrant (Q2) represent late apoptotic cells, those in the third quadrant (Q3) represent early apoptotic cells, whereas those in the fourth quadrant (Q4) represent normal cells. DIC: differential interference contrast; FITC: fluorescein isothiocyanate; PI: propidium iodide. **: P < 0.01. |
We accordingly observed that after 96 h of hypoxia stress, the TUNEL-positive signal (red) intensity of hepatocyte nuclei in the hypoxia group was significantly higher than that in the normoxia group (Fig. 2a). Likewise, after 24 h of hypoxia stress, the TUNEL-positive signal intensity of the nuclei of LYCF cells in the hypoxia group was significantly higher than that in the normoxia group (Fig. 2b).

|
| Fig.2 Changes in the TUNEL staining in the liver of Larimichthys crocea and LYCF cells after hypoxia stress a. the TUNEL results in L. crocea liver after 96 h of hypoxia stress; b. the TUNEL results in LYCF cells after 24 h of hypoxia stress. DAPI: 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride; TUNEL: TdT mediated dUTP nick end labelling. |
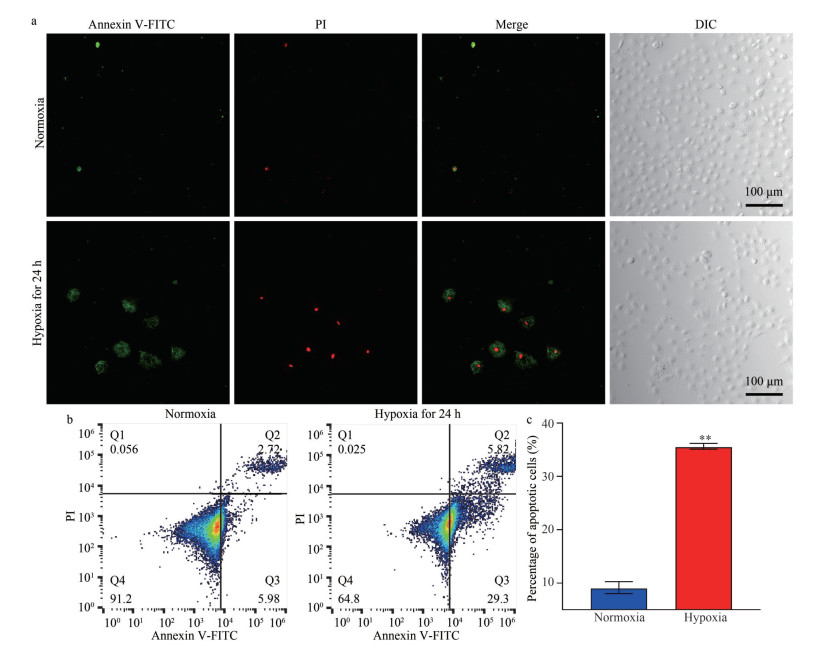
The ultrastructure of LYCF cells in the normoxia group is shown in Fig. 3a–b. We noticed that LYCF cells were oval in shape, with abundant mitochondria in the cytoplasm; mitochondrial cristae were clearly visible, while the nucleus was oval, containing a nucleolus in its center. After 24 h of hypoxia stress, we found that the nuclear membrane of LYCF cells was sunken inward, and some mitochondrial cristae in the cytoplasm were reduced or disappeared (Fig. 3c–d). The ultrastructure of LYCF cells after 48 h and 72 h of hypoxia stress is shown in Fig. 3e, f, & g–h. We specifically observed that the plasma membrane of LYCF cells was wrinkled. Compared with the group exposed to 24 h of hypoxia stress, the number of mitochondria with disappeared cristae was increased, and the inward depression of the nuclear membrane was more obvious in cells exposed to 48 h and 72 h of hypoxia stress.
|
| Fig.3 Ultrastructure of LYCF cells exposed to hypoxia a & b. the ultrastructure of LYCF cells in the normoxia group; c & d. the ultrastructure of LYCF cells after 24 h of hypoxia stress; e & f. the ultrastructure of LYCF cells after 48 h of hypoxia stress; g & h: the ultrastructure of LYCF cells after 72 h of hypoxia stress. N: nucleus, NO: nucleolus; M: mitochondria; MC: mitochondrial cristae. The red dashed ellipse shows the concave nuclear membrane, while the yellow dashed ellipse shows the folding of the plasma membrane. |
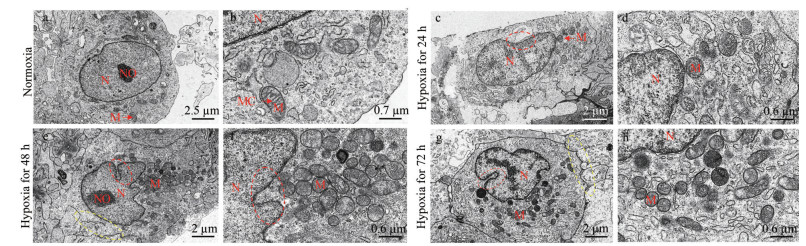
We found that after 24 h of hypoxia stress, the MMP (red fluorescence intensity) of the hypoxia group was significantly lower than that of the normoxia group (Fig. 4a). In addition, our flow cytometry analysis revealed that the relative mean fluorescence intensity (MFI) of TMRM in LYCF cells was significantly decreased after 24 h of hypoxia stress (Fig. 4b), indicating a significant decrease in the MMP in LYCF cells.
|
| Fig.4 Change of MMP in LYCF cells after 24 h of hypoxia stress a. laser confocal microscopy results of mitochondrial membrane potential (red signal) in LYCF cells after 24 h of hypoxia stress; b. flow cytometry of mitochondrial membrane potential in LYCF cells after 24 h of hypoxia stress. MFI: mean fluorescence intensity; DIC: differential interference contrast; TMRM: tetramethyl rhodamine methyl ester. **: P < 0.01. |
We noticed that the level of expression of Bax mRNA in the liver of the large yellow croaker was significantly increased under hypoxia stress (Fig. 5a). Meanwhile, the level of expression of Bcl-2 mRNA was significantly increased in the first 6 h of hypoxia stress and then rapidly decreased to a significantly lower level than that in the normoxia group (Fig. 5c). Correspondingly, we found that the Bax/Bcl-2 mRNA ratio showed an overall upward trend (Fig. 5e). We observed a similar trend in LYCF cells under hypoxia stress. The level of expression of Bax mRNA was significantly increased (Fig. 5b), whereas that of Bcl-2 mRNA was rapidly decreased to a significantly lower level than that in the normoxia group after an initial significant increase in the first 6 h of hypoxia stress (Fig. 5d), with the Bax/Bcl-2 mRNA ratio showing an overall upward trend (Fig. 5f). We also found that the expression of Casp3 mRNA was significantly increased in the liver and LYCF cells under hypoxia stress (Fig. 5g–h).
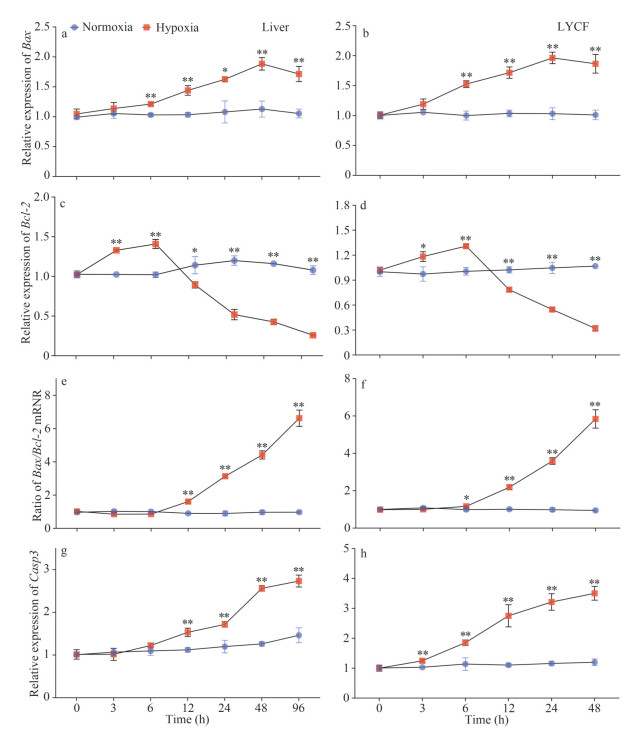
|
| Fig.5 Variations in the expression of Bax, Bcl-2, and Casp3 mRNA in the liver of Larimichthys crocea and LYCF cells under hypoxia stress a, c, & g. the variations in the expression of Bax, Bcl-2, and Casp3 mRNA in the liver of L. crocea under hypoxia stress, respectively; b, d, & h: the variations in the expression of Bax, Bcl-2, and Casp3 mRNA in LYCF cells under hypoxia stress, respectively; e. change in the Bax/Bcl-2 mRNA ratio in the liver of L. crocea under hypoxia stress; f. change in the Bax/Bcl-2 mRNA ratio in LYCF cells under hypoxia stress. Bax: Bcl-2 associated X; Bcl-2: B-cell lymphoma/leukemia-2; Casp3: caspase 3. *: P < 0.05; **: P < 0.01. |
We found that the cleavage activity (green fluorescence intensity) of Casp3/7 in LYCF cells after 24 h of hypoxia stress was significantly higher than that in the normoxia group (Fig. 6a). In addition, our flow cytometry analysis further showed that the cleavage activity of Casp3/7 (the relative MFI of GreenNucTM) was increased 2.24 folds in LYCF cells after 24 h of hypoxia stress compared with that in the normoxia group (Fig. 6b).

|
| Fig.6 Changes in the activity of caspase-3/7, caspase-3, caspase-8, and caspase-9 in the liver of Larimichthys crocea and LYCF cells under hypoxia stress a. laser confocal microscopy results of the activity of caspase-3/7 (green signal) in LYCF cells after 24 h of hypoxia stress; b. flow cytometry analysis results of the activity of caspase-3/7 in LYCF cells after 24 h of hypoxia stress; c, d, & e. the changes in the activity of caspase-3, caspase-8, and caspase-9 in the liver of L. crocea after 0, 3, 6, 12, 24, 48, and 96 h of hypoxia stress, respectively; f, g, & h. the changes in the activity of caspase-3, caspase-8, and caspase-9 in LYCF cells after 0, 1, 3, 6, 12, 24, and 48 h of hypoxia stress, respectively. *: P < 0.05; **: P < 0.01. |
Similar to our previous findings, we observed that the activity of Casp3, Casp8, and Casp9 was gradually increased in the liver during 96 h of hypoxia stress (Fig. 6c–e). Similarly, we detected that the activity of Casp3, Casp8, and Casp9 was gradually increased in LYCF cells during 48 h of hypoxia stress (Fig. 6f–h).
3.8 Change in the protein expression levels of Bcl-2 and AIF in the liver and LYCF cells under hypoxia stressChange in the levels of expression of the Bcl-2 and AIF proteins in the liver of L. crocea after hypoxia stress for 0, 3, 6, 12, 24, 48, and 96 h are shown in Fig. 7a, c, & e. We found that the expression level of the Bcl-2 protein showed an overall downward trend (Fig. 7a & c), whereas that of the AIF protein showed an overall upward trend (Fig. 7a & e) in the liver of L. crocea after 96 h of hypoxia stress.
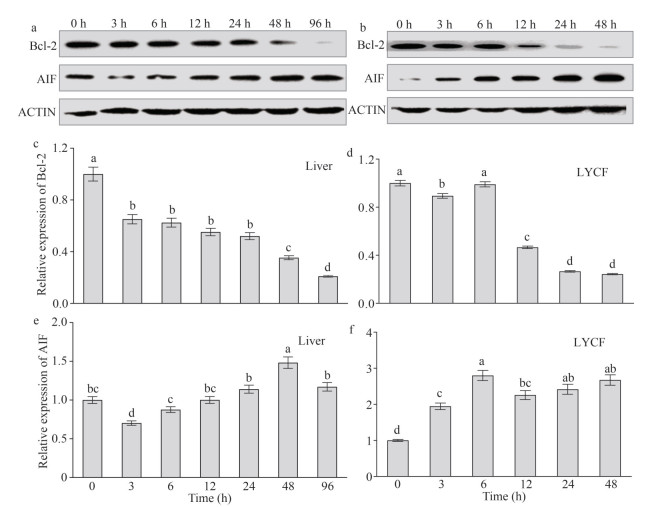
|
| Fig.7 Expression patterns of Bcl-2 and AIF proteins in the liver of Larimichthys crocea and LYCF cells under hypoxia stress a. changes in the levels of expression of the Bcl-2 and AIF proteins in the liver of L. crocea after 0, 3, 6, 12, 24, 48, and 96 h of hypoxia stress; b. changes in the levels of expression of the Bcl-2 and AIF proteins in LYCF cells after 0, 3, 6, 12, 24, and 48 h of hypoxia stress; c & e. the relative expression changes of Bcl-2 and AIF proteins, respectively, in the liver of L. crocea after hypoxia stress; d & f. the relative expression changes of Bcl-2 and AIF proteins, respectively, in LYCF cells after hypoxia stress. AIF: apoptosis-inducing factor; Bcl-2: B-cell lymphoma/leukemia-2. Different letters on the bars represent statistically significant intergroup differences (P < 0.05). |
In addition, change in the levels of expression of the Bcl-2 and AIF proteins in LYCF cells after hypoxia stress for 0, 3, 6, 12, 24, and 48 h are shown in Fig. 7a, d, & f. We specifically noticed that prolongation of hypoxia stress resulted in an overall downward trend in the level of expression of the Bcl-2 protein (Fig. 7b & d), whereas led to an overall upward trend in the level of expression of the AIF protein in LYCF cells (Fig. 7b & f).
3.9 Change in the nucleoplasmic distribution of AIF protein in the liver and LYCF cells under hypoxia stressChange in the expression levels of the AIF protein in the cytoplasm and nucleus of the liver after 96 h of hypoxia stress are shown in Fig. 8a. Compared with the normoxia group, we noticed that the AIF protein was significantly reduced in the cytoplasm, whereas significantly increased in the nucleus of cells in the hypoxia group. Changes in the expression levels of the AIF protein in the cytoplasm and nucleus of LYCF cells after 24 h of hypoxia stress are shown in Fig. 8b. Likewise, we detected that the AIF protein was significantly reduced in the cytoplasm, whereas significantly increased in the nucleus of LYCF cells in the hypoxia group compared with those in the normoxia group. Our immunofluorescence (IF) analysis further revealed the stronger presence of AIF in the nuclei of cells in the hypoxia group (Fig. 8c).
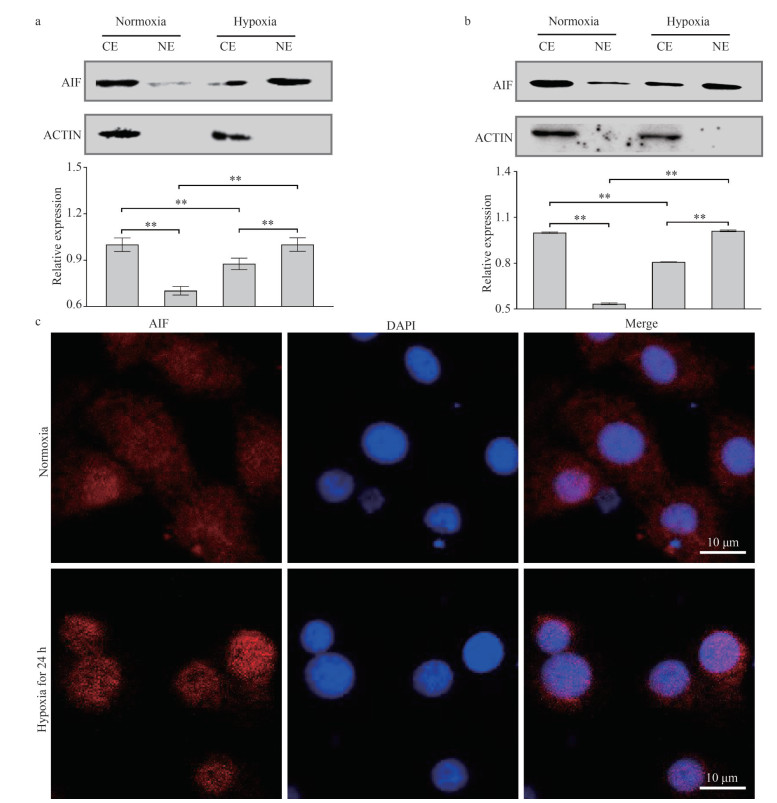
|
| Fig.8 Changes in the levels of AIF protein in the cytoplasm and nuclei in the liver of Larimichthys crocea and LYCF cells under hypoxia stress a. changes in the level of AIF protein in the cytoplasm and nuclei in the liver of L. crocea after 96 h of hypoxia stress; b. changes in the levels of AIF protein in the cytoplasm and nuclei of LYCF cells after 24 h of hypoxia stress; c. immunofluorescence results of AIF in LYCF cells after 24 h of hypoxia stress. **: P < 0.01. CE: expression in the cytoplasm; NE: expression in the nuclei; DAPI: 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride. |
A number of studies have investigated the hypoxia-induced apoptosis and its underlying molecular mechanism in higher animals (Green and Reed, 1998; Murphy et al., 1999; Kunz et al., 2001; Wang et al., 2020a, 2021). Given the frequent occurrence of marine hypoxia, investigating the effect of hypoxia stress on the apoptosis in L. crocea can provide important basic biological information for elucidating the mechanism of hypoxia response in marine organisms. In this study, we found that environmental hypoxia induced apoptosis both in vivo and in vitro in L. crocea through the mitochondrial and death receptor pathways.
4.1 Hypoxia stress induced apoptosis both in vivo and in vitro in L. croceaHypoxia stress is one of the factors known to induce apoptosis in terrestrial animals (Zhang et al., 2013; Pan et al., 2014; Sendoel and Hengartner, 2014; Lohberger et al., 2016). Due to the particularity of their respiratory system and habitat, fish might encounter more frequent hypoxia stresses than terrestrial animals. Therefore, the correlation of apoptosis with the response of fish under hypoxia stress has been of special interest to researchers in recent years. Wu et al. (2016) and Ding et al. (2018) examined the apoptotic levels of cardiomyocytes in M. amblycephala and H. molitrix under hypoxia stress using the TUNEL method, and found that hypoxia stress induced significant levels of apoptosis in the cardiomyocytes of both fish species. Likewise, Zhao et al. (2016) examined the liver and brain of H. molitrix under hypoxia stress using annexin V-FITC/PI flow cytometry and the TUNEL assay, and discovered that hypoxia stress induced apoptosis in both the liver and brain of H. molitrix. To date, no studies have been reported on the effects of hypoxia stress on apoptosis in L. crocea. In this study, we detected for the first time the apoptotic levels in the liver of L. crocea and LYCF cells after 24 and 96 h of hypoxia stress, respectively, using annexin V-FITC/PI flow cytometry and the TUNEL assay. Our results show that the apoptotic rate of LYCF cells in the hypoxia group was significantly higher than that in the normoxia group. In particular, the number of LYCF cells in late apoptosis was significantly higher in the hypoxia group than that in the normoxia group. Similarly, we found that the number of cells in late apoptosis in the liver of fish in the hypoxia group was significantly higher than that in the normoxia group. These results suggested that hypoxia stress induced apoptosis in L. crocea both in vivo and in vitro, suggesting that apoptosis might be a common response of animals to hypoxia stress.
In 1972, Kerr et al. (1972) first reported the morphological characteristics of apoptotic cells, including cell shrinkage, intact but wrinkled cell membranes, constricted nuclei, condensed nuclear chromatin around the nuclear membrane, and eventual dissolution of cells into several apoptotic bodies with intact membrane structures. In this study, we evaluated the ultrastructure of LYCF cells after 24, 48, and 72 h of hypoxia stress, respectively. We accordingly observed that compared with the normoxia group, the hypoxia group of LYCF cells exhibited the following features: wrinkling of the plasma membrane, constriction of the nuclear membrane by inward depression, breakage or disappearance of some mitochondrial cristae in the cytoplasm, condensation of nuclear chromatin, fragmentation and aggregation around the nuclear membrane. Concomitantly, we found that mitochondrial cristae were broken, mitochondria were vacuolated, nuclei were deformed, and the chromatin was concentrated and fragmented around the nuclear membrane in liver tissue cells after 96 h of hypoxia stress (Luo et al., 2021). These findings further suggested that hypoxia stress induced apoptosis in the liver of L. crocea and LYCF cells. In accordance to our findings, Sollid et al. (2003) observed cell shrinkage and chromatin fragmentation around the nuclear membrane in the gill tissue of Carassius carassius after hypoxia stress.
4.2 Hypoxia stress induced apoptosis in L. crocea through the mitochondrial pathwayThe mitochondrial pathway is one of the major pathways of hypoxia-induced apoptosis (Pan et al., 2014; Lohberger et al., 2016). The oligomerization of Bax and Bak in the mitochondria-mediated apoptotic pathway has been reported to induce a decrease in MMP and an increase in outer mitochondrial membrane permeability (Knudson et al., 1995; Wei et al., 2001; Reed, 2006). In this study, we identified that hypoxia stress caused a decrease in MMP in LYCF cells. Combined with the observed in vivo and in vitro induced apoptosis of cells, we postulated that the decrease in MMP was one of the reasons for the occurrence of apoptosis under hypoxia stress. The hypoxia stress-induced reduction of cellular MMP has also been reported in human pulmonary artery smooth muscle cells (hPASMCs) (Hu et al., 2010), human colorectal cancer cells (HCT116) (Dong et al., 2019), hepatocellular carcinoma cells (HCC) (Cai et al., 2019), and human retinal pigment epithelial cells (Feng et al., 2020). The Bcl-2 protein is mainly located in the mitochondrial membrane, playing a role in antagonizing Bax. The increased expression of Bcl-2 is known to results in the polymerization of a large amount of Bcl-2 with Bax toward the formation of a heterodimer, thus preventing the oligomeric activation of Bax and Bak. In contrast, and despite the formation of a heterodimer with Bcl-2, the increased expression of Bax results in the excess Bax continuing to play its pro-apoptotic role with the oligomeric activation of Bak. Therefore, the ratio of the expression of the Bax/Bcl-2 genes has been used to indicate the level of intrinsic apoptosis (Tsukahara et al., 2006). In this study, we noticed the upregulated expression of Bax in the liver of L. crocea and LYCF cells under hypoxia stress. Whereas, the expression of Bcl-2 was shown to be downregulated, with the Bax/Bcl-2 ratio being accordingly significantly increased. Studies on the hypoxia stress-induced elevated Bax/Bcl-2 mRNA ratios in fish have also been reported in M. amblycephala (Wu et al., 2016), Ictalurus punctatus (Yuan et al., 2016), and H. molitrix (Ding et al., 2018). In addition, the present study revealed the significantly reduced expression of Bcl-2 in the liver of L. crocea and LYCF cells under hypoxia stress. Similar results were observed in studies of primary cultured rat cortical neurons (Tamatani et al., 1998) and rat cardiomyocytes (Li et al., 2011). The enhanced mitochondrial outer membrane permeability has been shown to lead to the release of CytC from the mitochondria into the cytosol (Liu et al., 1996; Ow et al., 2008) to bind to Apaf1 and activate Caspase-9 (Yuan and Akey, 2013). Activated caspase-9 cleaves procaspase-3 and procaspase-7 into active caspase-3 and caspase-7, respectively, which in turn triggers apoptosis (Acehan et al., 2002). Here, we found that the activity of caspase-9 and caspase-3/7 was significantly elevated in the liver of L. crocea and LYCF cells under hypoxia stress. Similarly, the activity of caspase-9 was reported to be significantly increased in the cytoplasm of human corneal epithelial cells (Kurpakus-Wheater et al., 2003), neonatal piglet cerebral cortex cells (Mishra and Delivoria-Papadopoulos, 2006), and mitochondria of neonatal piglet cerebral cortex (Mishra et al., 2006). The hypoxia stress-induced activation of caspase-3/7 has also been reported in Carassius auratus (Poli et al., 2003), D. rerio (Williams et al., 2017) and M. undulatus (Ondricek and Thomas, 2018). The above findings suggested that hypoxia stress induces the mitochondrial pathway-mediated caspase-dependent apoptosis in L. crocea.
In addition to CytC, the AIF pro-apoptotic protein has also been shown to translocate from the mitochondria to the cytoplasm and then to the nucleus following the altered mitochondrial membrane permeability, promoting the fragmentation of genomic DNA for the execution of a caspase-independent apoptotic program (Susin et al., 1999). Studies have shown that hypoxia can induce an increase in the level of expression of the AIF protein (Goel et al., 2010; Kim et al., 2017). In this study, the expression levels of the AIF protein were significantly increased in the liver of L. crocea and LYCF cells under hypoxia stress; moreover, hypoxia stress induced the nuclear translocation of the AIF protein. Studies on the hypoxia stress-induced nuclear translocation of AIF have also been reported in higher mammals. For example, Brukamp et al. (2007) found that the nuclear translocation of the AIF protein was significantly enhanced in mouse podocytes under hypoxia stress. Likewise, Kim et al. (2010) found that the nuclear translocation of the AIF protein was significantly augmented in human lung adenocarcinoma cells (A549) after hypoxic hypoglycemic stress. These investigators suggested that the release of AIF from the cytoplasm into the nucleus for the promotion of DNA fragmentation is one of the pathways through which hypoxia stress induces apoptosis. Therefore, we postulated that hypoxia stress induces the AIF-mediated caspase-independent apoptosis in L. crocea.
4.3 Hypoxia stress induced apoptosis in L. crocea through the death receptor pathwayThe extrinsic apoptotic pathway is known to be activated by death ligands such as FasL, TNF-α, and TRAIL, which bind to specific receptors on the cell surface to generate DISC, which cleaves procaspase-8 into active caspase-8 (Ashkenazi and Dixit, 1998). In turn, the activated caspase-8 cleave procaspase-3 to its active caspase-3 form that executes the apoptotic process (Tummers and Green, 2017). The activated caspase-8 has also been shown to cleave Bid into its active truncated Bid form, which rapidly moves to the outer mitochondrial membrane, promoting the oligomerization of Bax and Bak for the opening of the mitochondrial membrane permeability translocation pore and the formation of the pro-apoptotic protein release channel (Cassidy-Stone et al., 2008). Studies on the hypoxia stress-induced enhancement of the activity of caspase-8 have been reported in rat ventricular myocytes (Gurevich et al., 2001), human cardiac myocytes (Chao et al., 2002), neonatal piglet cerebral cortex (Khurana et al., 2002), and oral cancer cells (Nagarajah et al., 2004). In this study, we examined the activity of caspase-8 in the liver of L. crocea and LYCF cells during 96 and 48 h of hypoxia stress, respectively, and found that hypoxia stress significantly enhanced the activity of caspase-8 both in vivo and in vitro. These findings suggested that hypoxia induced the death receptor pathway-mediated apoptosis in L. crocea.
5 CONCLUSIONIn this study, we performed annexin V-FITC/PI, TUNEL, and TEM assays to show that hypoxia stress induced apoptosis in L. crocea both in vivo and in vitro. Analyses of MMP, of the activity of caspase-3/7/9, of the level of expression of the Bcl-2 protein, of the Bax/Bcl-2 mRNA ratio, and of the expression of Casp3 mRNA indicated that hypoxia stress induced the mitochondrial pathway-mediated apoptosis in L. crocea. More specifically, analysis of the level of expression of the AIF protein and its nucleoplasmic distribution indicated that hypoxia stress induced the mitochondria/AIF-mediated caspase-independent apoptosis in L. crocea. Finally, analysis of the activity of caspase-8 revealed that hypoxia stress also induced the extrinsic pathway-mediated apoptosis in L. crocea. In summary, we found that hypoxia stress can induce apoptosis in L. crocea through both the intrinsic and extrinsic pathways.
6 DATA AVAILABILITY STATEMENTThe data of this study are available from the corresponding author upon reasonable request.
Acehan D, Jiang X J, Morgan D G, et al. 2002. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Molecular Cell, 9(2): 423-432.
DOI:10.1016/S1097-2765(02)00442-2 |
Ashkenazi A, Dixit V M. 1998. Death receptors: signaling and modulation. Science, 281(5381): 1305-1308.
DOI:10.1126/science.281.5381.1305 |
Breitburg D, Levin L A, Oschlies A, et al. 2018. Declining oxygen in the global ocean and coastal waters. Science, 359(6371): eaam7240.
DOI:10.1126/science.aam7240 |
Brukamp K, Jim B, Moeller M J, et al. 2007. Hypoxia and podocyte-specific Vhlh deletion confer risk of glomerular disease. American Journal of Physiology Renal Physiology, 293(4): F1397-F1407.
DOI:10.1152/ajprenal.00133.2007 |
Cai M, He P, Fang D L. 2019. Hypoxia‑induced mitochondrial translocation of DNM1L increases mitochondrial fission and triggers mPTP opening in HCC cells via activation of HK2. Oncology Reports, 42(3): 1125-1132.
|
Cassidy-Stone A, Chipuk J E, Ingerman E, et al. 2008. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Developmental Cell, 14(2): 193-204.
DOI:10.1016/j.devcel.2007.11.019 |
Chao D T, Korsmeyer S J. 1998. BCL-2 FAMILY: regulators of cell death. Annual Review of Immunology, 16: 395-419.
DOI:10.1146/annurev.immunol.16.1.395 |
Chao W, Shen Y, Li L, et al. 2002. Importance of FADD signaling in serum deprivation-and hypoxia-induced cardiomyocyte apoptosis. Journal of Biological Chemistry, 277(35): 31639-31645.
DOI:10.1074/jbc.M204104200 |
Crompton M. 1999. The mitochondrial permeability transition pore and its role in cell death. Biochemical Journal, 341(2): 233-249.
DOI:10.1042/bj3410233 |
Diaz R J. 2001. Overview of hypoxia around the world. Journal of Environmental Quality, 30(2): 275-281.
DOI:10.2134/jeq2001.302275x |
Ding C Y, Hu L S, Li Y, et al. 2018. Effects of hypoxia stress on cardiomyocyte apoptosis and the control for Bax, Bcl-2 expressions in Hypophthalmichthys molitrix. Freshwater Fisheries, 48(2): 10-15.
(in Chinese with English abstract) DOI:10.3969/j.issn.1000-6907.2018.02.002 |
Dong Y, Wu Y, Zhao G L, et al. 2019. Inhibition of autophagy by 3-MA promotes hypoxia-induced apoptosis in human colorectal cancer cells. European Review for Medical and Pharmacological Sciences, 23(3): 1047-1054.
|
Feng J Y, Tan W, Li T, et al. 2020. Human retinal pigment epithelial cells are protected against hypoxia by BNIP3. Annals of Translational Medicine, 8(22): 1502.
DOI:10.21037/atm-20-7145 |
Goel G, Guo M, Ding J, et al. 2010. Combined effect of tumor necrosis factor (TNF)-α and heat shock protein (HSP)-70 in reducing apoptotic injury in hypoxia: a cell culture study. Neuroscience Letters, 483(3): 162-166.
DOI:10.1016/j.neulet.2010.07.069 |
Green D R, Reed J C. 1998. Mitochondria and apoptosis. Science, 281(5381): 1309-1312.
DOI:10.1126/science.281.5381.1309 |
Grilo A L, Mantalaris A. 2019. Apoptosis: a mammalian cell bioprocessing perspective. Biotechnology Advances, 37(3): 459-475.
DOI:10.1016/j.biotechadv.2019.02.012 |
Gurevich R M, Regula K M, Kirshenbaum L A. 2001. Serpin protein CrmA suppresses hypoxia-mediated apoptosis of ventricular myocytes. Circulation, 103(15): 1984-1991.
DOI:10.1161/01.CIR.103.15.1984 |
Halliwell B. 1992. Reactive oxygen species and the central nervous system. Journal of Neurochemistry, 59(5): 1609-1623.
DOI:10.1111/j.1471-4159.1992.tb10990.x |
Hammond E M, Denko N C, Dorie M J, et al. 2002. Hypoxia links ATR and p53 through replication arrest. Molecular and Cellular Biology, 22(6): 1834-1843.
DOI:10.1128/MCB.22.6.1834-1843.2002 |
Hausenloy D J, Duchen M R, Yellon D M. 2003. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovascular Research, 60(3): 617-625.
DOI:10.1016/j.cardiores.2003.09.025 |
Hu H L, Zhang Z X, Chen C S, et al. 2010. Effects of mitochondrial potassium channel and membrane potential on hypoxic human pulmonary artery smooth muscle cells. American Journal of Respiratory cell and Molecular Biology, 42(6): 661-666.
DOI:10.1165/rcmb.2009-0017OC |
Kerr J F R, Wyllie A H, Currie A R. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer, 26(4): 239-257.
DOI:10.1038/bjc.1972.33 |
Khurana P, Ashraf Q M, Mishra O P, et al. 2002. Effect of hypoxia on caspase-3, -8, and -9 activity and expression in the cerebral cortex of newborn piglets. Neurochemical Research, 27(9): 931-938.
DOI:10.1023/A:1020347732741 |
Kim C H, Ko A R, Lee S Y, et al. 2010. Hypoxia switches glucose depletion-induced necrosis to phosphoinositide 3-kinase/Akt-dependent apoptosis in A549 lung adenocarcinoma cells. International Journal of Oncology, 36(1): 117-124.
|
Kim Y, Kim Y S, Noh M Y, et al. 2017. Neuroprotective effects of a novel poly (ADP-ribose) polymerase-1 inhibitor, JPI-289, in hypoxic rat cortical neurons. Clinical and Experimental Pharmacology and Physiology, 44(6): 671-679.
DOI:10.1111/1440-1681.12757 |
Knudson C M, Tung K S K, Tourtellotte W G, et al. 1995. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science, 270(5233): 96-99.
DOI:10.1126/science.270.5233.96 |
Kunz M, Ibrahim S, Koczan D, et al. 2001. Activation of c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) is critical for hypoxia-induced apoptosis of human malignant melanoma. Cell Growth & Differentiation, 12(3): 137-145.
|
Kurpakus-Wheater M, Sexton R, McDermott M L, et al. 2003. Caspase-9 activation in hypoxic human corneal epithelial cells. Apoptosis, 8(6): 681-688.
DOI:10.1023/A:1026164332473 |
Li J Y, Dai H, Liu H, et al. 2011. Effects of scutellarin benzyl ester on the expressions of Bcl-2 and Bax in cardiomyocytes injured by acute hypoxia. Chinese Critical Care Medicine, 23(6): 337-340.
(in Chinese with English abstract) DOI:10.3760/cma.j.issn.1003-0603.2011.06.006 |
Li Y L, Xu G Y, Xiao J W, et al. 2017. Studies on the protective role of zebrafish HO1 in response to hypoxia. Acta Hydrobiologica Sinica, 41(1): 43-49.
(in Chinese with English abstract) |
Liu X S, Kim C N, Yang J, et al. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell, 86(1): 147-157.
DOI:10.1016/S0092-8674(00)80085-9 |
Lohberger B, Steinecker-Frohnwieser B, Stuendl N, et al. 2016. The proteasome inhibitor bortezomib affects chondrosarcoma cells via the mitochondria-caspase dependent pathway and enhances death receptor expression and autophagy. PLoS One, 11(12): e0168193.
DOI:10.1371/journal.pone.0168193 |
Lu G, Mak Y T, Wai S M, et al. 2005. Hypoxia-induced differential apoptosis in the central nervous system of the sturgeon (Acipenser shrenckii). Microscopy Research and Technique, 68(5): 258-263.
DOI:10.1002/jemt.20243 |
Luo S Y, Gao X M, Ding J, et al. 2019. Transcriptome sequencing reveals the traits of spermatogenesis and testicular development in large yellow croaker (Larimichthys crocea). Genes, 10(12): 958.
DOI:10.3390/genes10120958 |
Martínez M L, Raynard E L, Rees B B, et al. 2011. Oxygen limitation and tissue metabolic potential of the African fish Barbus neumayeri: roles of native habitat and acclimatization. BMC Ecology, 11(1): 1-9.
DOI:10.1186/1472-6785-11-1 |
Mishra O P, Delivoria-Papadopoulos M. 2006. Effect of neuronal nitric oxide synthase inhibition on caspase-9 activity during hypoxia in the cerebral cortex of newborn piglets. Neuroscience Letters, 401(1-2): 81-85.
DOI:10.1016/j.neulet.2006.02.070 |
Mishra O P, Randis T, Ashraf Q M, et al. 2006. Hypoxia-induced Bax and Bcl-2 protein expression, caspase-9 activation, DNA fragmentation, and lipid peroxidation in mitochondria of the cerebral cortex of newborn piglets: the role of nitric oxide. Neuroscience, 141(3): 1339-1349.
DOI:10.1016/j.neuroscience.2006.05.005 |
Murphy A N, Fiskum G, Beal M F. 1999. Mitochondria in neurodegeneration: bioenergetic function in cell life and death. Journal of Cerebral Blood Flow & Metabolism, 19(3): 231-245.
|
Nagarajah N S, Vigneswaran N, Zacharias W. 2004. Hypoxia-mediated apoptosis in oral carcinoma cells occurs via two independent pathways. Molecular Cancer, 3(1): 38.
DOI:10.1186/1476-4598-3-38 |
Ondricek K, Thomas P. 2018. Effects of hypoxia exposure on apoptosis and expression of membrane steroid receptors, ZIP9, mPRα, and GPER in Atlantic croaker ovaries. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 224: 84-92.
|
Ow Y L P, Green D R, Hao Z Y, et al. 2008. Cytochrome c: functions beyond respiration. Nature Reviews Molecular Cell Biology, 9(7): 532-542.
DOI:10.1038/nrm2434 |
Pan W L, Wong J H, Fang E F, et al. 2014. Preferential cytotoxicity of the type Ⅰ ribosome inactivating protein alpha-momorcharin on human nasopharyngeal carcinoma cells under normoxia and hypoxia. Biochemical Pharmacology, 89(3): 329-339.
DOI:10.1016/j.bcp.2014.03.004 |
Poli A, Beraudi A, Villani L, et al. 2003. Group Ⅱ metabotropic glutamate receptors regulate the vulnerability to hypoxic brain damage. The Journal of Neuroscience, 23(14): 6023-6029.
DOI:10.1523/JNEUROSCI.23-14-06023.2003 |
Quignard S, Mosser G, Boissière M, et al. 2012. Long-term fate of silica nanoparticles interacting with human dermal fibroblasts. Biomaterials, 33(17): 4431-4442.
DOI:10.1016/j.biomaterials.2012.03.004 |
Reed J C. 2006. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death & Differentiation, 13(8): 1378-1386.
|
Ren G X, Guo W, Ye D X, et al. 2006. A study on the mechanism of inducing apoptosis of Tca8113 cells by means of ultrasound hyperthermia. Shanghai Journal of Stomatology, 15(5): 507-511.
(in Chinese with English abstract) DOI:10.3969/j.issn.1006-7248.2006.05.015 |
Ren Q Y, Zhang M Z, Li M, et al. 2018. Differential induction of gene expressions, protein contents and enzyme activities involved in hypoxic responsive in liver tissues of mudskipper Boleophthalmus pectinirostris exposed to acute hypoxia. Oceanologia et Limnologia Sinica, 49(4): 889-896.
(in Chinese with English abstract) |
Schulte P M. 2014. What is environmental stress? Insights from fish living in a variable environment. Journal of Experimental Biology, 217(1): 23-34.
DOI:10.1242/jeb.089722 |
Semenza G L. 2011. Oxygen sensing, homeostasis, and disease. The New England Journal of Medicine, 365(6): 537-547.
DOI:10.1056/NEJMra1011165 |
Sendoel A, Hengartner M O. 2014. Apoptotic cell death under hypoxia. Physiology, 29(3): 168-176.
DOI:10.1152/physiol.00016.2013 |
Sollid J, De Angelis P, Gundersen K, et al. 2003. Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. Journal of Experimental Biology, 206(20): 3667-3673.
DOI:10.1242/jeb.00594 |
Sun C F, Tao Y, Jiang X Y, et al. 2011. IGF binding protein 1 is correlated with hypoxia-induced growth reduce and developmental defects in grass carp (Ctenopharyngodon idellus) embryos. General and Comparative Endocrinology, 172(3): 409-415.
DOI:10.1016/j.ygcen.2011.04.005 |
Susin S A, Lorenzo H K, Zamzami N, et al. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature, 397(6718): 441-446.
DOI:10.1038/17135 |
Tamatani M, Ogawa S, Tohyama M. 1998. Roles of Bcl-2 and caspases in hypoxia-induced neuronal cell death: a possible neuroprotective mechanism of peptide growth factors. Molecular Brain Research, 58(1-2): 27-39.
DOI:10.1016/S0169-328X(98)00095-3 |
Tse A C K, Li J W, Chan T F, et al. 2015. Hypoxia induces miR-210, leading to anti-apoptosis in ovarian follicular cells of marine medaka Oryzias melastigma. Aquatic Toxicology, 165: 189-196.
DOI:10.1016/j.aquatox.2015.06.002 |
Tsukahara S, Yamamoto S, Shwe T T W, et al. 2006. Inhalation of low-level formaldehyde increases the Bcl-2/Bax expression ratio in the hippocampus of immunologically sensitized mice. Neuroimmunomodulation, 13(2): 63-68.
DOI:10.1159/000094829 |
Tummers B, Green D R. 2017. Caspase-8:regulating life and death. Immunological Reviews, 277(1): 76-89.
DOI:10.1111/imr.12541 |
Vuori K A M, Soitamo A, Vuorinen P J, et al. 2004. Baltic salmon (Salmo salar) yolk-sac fry mortality is associated with disturbances in the function of hypoxia-inducible transcription factor (HIF-1α) and consecutive gene expression. Aquatic Toxicology, 68(4): 301-313.
DOI:10.1016/j.aquatox.2004.03.019 |
Wang J C, Xue Z M, Hua C T, et al. 2020a. Metabolomic analysis of the ameliorative effect of enhanced proline metabolism on hypoxia-induced injury in cardiomyocytes. Oxidative Medicine and Cellular Longevity, 2020: 8866946.
|
Wang M X, Rong Y, Luo L. 2021. Neuroprotective effects of icariin in neonatal hypoxia-ischemic brain damage via its anti-apoptotic property. Child's Nervous System, 37(1): 39-46.
DOI:10.1007/s00381-020-04690-8 |
Wang X H, Li Q H, Mu P F, et al. 2020b. Large yellow croaker peroxiredoxin Ⅳ protect cells against oxidative damage and apoptosis. Molecular Immunology, 127: 150-156.
DOI:10.1016/j.molimm.2020.08.019 |
Wang Y, Liu C X, Yi Y R, et al. 2006. Detection of apoptosis of the human hepatic carcinoma cells induced by bluetongus virus by PI and Annexin V/PI staining methods. Virologica Sinica, 21(3): 253-256.
(in Chinese with English abstract) |
Wei M C, Zong W X, Cheng E H Y, et al. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science, 292(5517): 727-730.
|
Williams T A, Bergstrome J C, Scott J, et al. 2017. CRF and urocortin 3 protect the heart from hypoxia/reoxygenation-induced apoptosis in zebrafish. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 313(2): R91-R100.
|
Wu X J, Chen N, Huang C X, et al. 2016. Effects of hypoxia on cardiomyocyte apoptosis and activity of antioxidant enzymes in Megalobrama amblycephala heart. Journal of Huazhong Agricultural University, 35(3): 108-113.
(in Chinese with English abstract) |
Youle R J, Strasser A. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nature Reviews Molecular Cell Biology, 9(1): 47-59.
|
Yuan S J, Akey C W. 2013. Apoptosome structure, assembly, and procaspase activation. Structure, 21(4): 501-515.
|
Yuan Z H, Liu S K, Yao J, et al. 2016. Expression of Bcl-2 genes in channel catfish after bacterial infection and hypoxia stress. Developmental & Comparative Immunology, 65: 79-90.
|
Zhang S W, Zhao Y L, Xu M, et al. 2013. FoxO3a modulates hypoxia stress induced oxidative stress and apoptosis in cardiac microvascular endothelial cells. PLoS One, 8(11): e80342.
|
Zhao J K, Liang H W, Zou G W, et al. 2016. Influence of hypoxic stress on apoptosis of hepatocyte and brain cells of silver carp (Hypophthalmichthys molitrix). Journal of Northwest A & F University (Natural Science Edition), 44(7): 34-38.
(in Chinese with English abstract) |
Zhao L L, Cui C, Liu Q, et al. 2020. Combined exposure to hypoxia and ammonia aggravated biological effects on glucose metabolism, oxidative stress, inflammation and apoptosis in largemouth bass (Micropterus salmoides). Aquatic Toxicology, 224: 105514.
|
 2023, Vol. 41
2023, Vol. 41